Antacid Tablet Chemical Equation . What is the formula for an antacid? The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. The base (the antacid) turns the acid primarily into salt and water. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Commercial antacids consist of a number of ingredients, such as binders and flavorings, but the active ingredient is simply a basic salt such. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the.
from www.numerade.com
Commercial antacids consist of a number of ingredients, such as binders and flavorings, but the active ingredient is simply a basic salt such. The base (the antacid) turns the acid primarily into salt and water. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. What is the formula for an antacid?
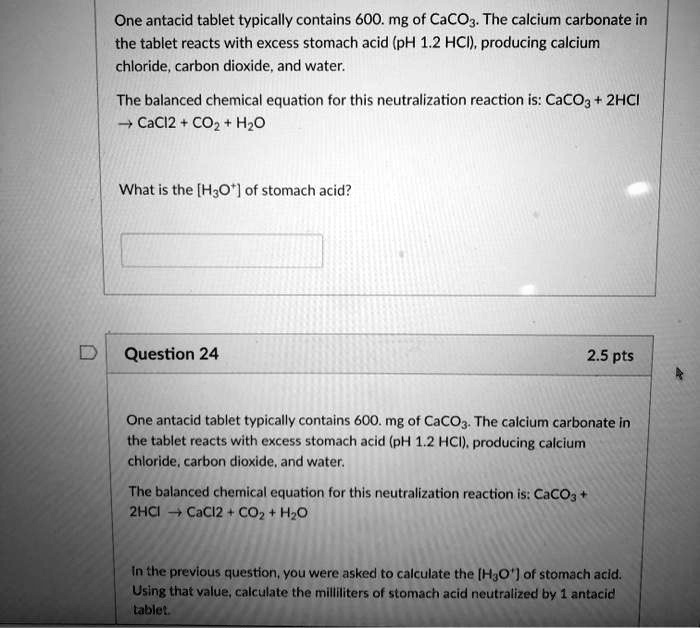
One antacid tablet typically contains 600 mg of CaCO3. The calcium
Antacid Tablet Chemical Equation What is the formula for an antacid? In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. The base (the antacid) turns the acid primarily into salt and water. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. Commercial antacids consist of a number of ingredients, such as binders and flavorings, but the active ingredient is simply a basic salt such. What is the formula for an antacid?
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Antacid Tablet Chemical Equation The base (the antacid) turns the acid primarily into salt and water. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. Although equation 2 shows that antacids can neutralize stomach acid, just how. Antacid Tablet Chemical Equation.
From www.chegg.com
Solved One antacid tablet typically contains 600.mg of Antacid Tablet Chemical Equation The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Commercial antacids consist of a number. Antacid Tablet Chemical Equation.
From www.chegg.com
An antacid tablet contains citric acid and sodium Antacid Tablet Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon. Antacid Tablet Chemical Equation.
From renatatara.blogspot.com
Antacid Tablet Chemical Formula An Antacid Tablet Contains Citric Antacid Tablet Chemical Equation A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. What is the formula for an antacid? The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets. Antacid Tablet Chemical Equation.
From renatatara.blogspot.com
Antacid Tablet Chemical Formula An Antacid Tablet Contains Citric Antacid Tablet Chemical Equation \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. What is the formula for an antacid? Commercial antacids consist of a number of ingredients, such as binders and flavorings, but the active ingredient is simply a basic salt such. The base (the antacid) turns the acid primarily into salt and water. The average therapeutic dose of antacid is 10 to 15 ml (1. Antacid Tablet Chemical Equation.
From maxfacts.uk
Antacid medications Antacid Tablet Chemical Equation Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. What is the formula for an antacid? \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the.. Antacid Tablet Chemical Equation.
From talisman-intl.com
🎉 Antacid chemical reaction. Chemistry 104 Analysis of An Antacid Antacid Tablet Chemical Equation A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. In this experiment, several brands of antacids. Antacid Tablet Chemical Equation.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID4269478 Antacid Tablet Chemical Equation The base (the antacid) turns the acid primarily into salt and water. What is the formula for an antacid? \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. Commercial antacids consist of a number of ingredients, such as binders and. Antacid Tablet Chemical Equation.
From www.youtube.com
Antacid Titration Calculation YouTube Antacid Tablet Chemical Equation The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. What is the formula for an antacid?. Antacid Tablet Chemical Equation.
From www.numerade.com
SOLVED Data Antacid 1 Antacid 2 Brand of antacid tablet TUMs TUMs Mass Antacid Tablet Chemical Equation The base (the antacid) turns the acid primarily into salt and water. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. Commercial antacids consist of a number of ingredients, such as binders and. Antacid Tablet Chemical Equation.
From www.numerade.com
SOLVED Write balanced chemical equation for the reaction of your Antacid Tablet Chemical Equation The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. The base (the antacid) turns the acid primarily into salt and water. What is the formula for an antacid? Commercial antacids consist of a number of ingredients, such as binders. Antacid Tablet Chemical Equation.
From valentin-has-decker.blogspot.com
Antacid Tablet Chemical Formula ValentinhasDecker Antacid Tablet Chemical Equation \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. What is the formula for an antacid? In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost.. Antacid Tablet Chemical Equation.
From talisman-intl.com
🎉 Antacid chemical reaction. Chemistry 104 Analysis of An Antacid Antacid Tablet Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. Commercial antacids consist of a number of. Antacid Tablet Chemical Equation.
From www.academia.edu
(PDF) AcidBase Analysis of Antacid Tablets asmha asmha123 Academia.edu Antacid Tablet Chemical Equation \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day.. Antacid Tablet Chemical Equation.
From meryngross.blogspot.com
Antacid Tablet Chemical Formula MerynGross Antacid Tablet Chemical Equation \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. The base (the antacid) turns the acid primarily into salt and water. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. What is the formula for an antacid? Although equation 2 shows that. Antacid Tablet Chemical Equation.
From sites.google.com
Neutralization MissReyes8thgradeScience Antacid Tablet Chemical Equation The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. In. Antacid Tablet Chemical Equation.
From studylib.net
The Neutralizing Ability of Antacid Tablets Lab thsicp23 Antacid Tablet Chemical Equation A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. Commercial antacids consist of a number of. Antacid Tablet Chemical Equation.
From www.numerade.com
SOLVED Several brands of antacids use Al(OH)3 to react with stomach Antacid Tablet Chemical Equation The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. What is the formula for an antacid? In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the. Antacid Tablet Chemical Equation.
From valentin-has-decker.blogspot.com
Antacid Tablet Chemical Formula ValentinhasDecker Antacid Tablet Chemical Equation The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. The base (the antacid) turns the. Antacid Tablet Chemical Equation.
From www.numerade.com
Bubbles appear as hydrochloric acid is poured ont… Antacid Tablet Chemical Equation A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. Commercial antacids consist of a number of ingredients, such as binders and flavorings, but. Antacid Tablet Chemical Equation.
From www.slideshare.net
Chem unit 10 presentation Antacid Tablet Chemical Equation The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. The base (the antacid) turns the acid primarily into salt and water. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise. Antacid Tablet Chemical Equation.
From www.numerade.com
Antacid Fizz When an antacid tablet dissolves in water, the fizz is due Antacid Tablet Chemical Equation \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. The base (the antacid) turns the acid primarily into salt and water. Commercial antacids consist of a number of ingredients, such as binders and flavorings, but the active ingredient is simply a basic salt such. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized. Antacid Tablet Chemical Equation.
From renatatara.blogspot.com
Antacid Tablet Chemical Formula An Antacid Tablet Contains Citric Antacid Tablet Chemical Equation The base (the antacid) turns the acid primarily into salt and water. Commercial antacids consist of a number of ingredients, such as binders and flavorings, but the active ingredient is simply a basic salt such. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. What is the formula for an antacid? A simple antacid tablet, milk of magnesia, has the following active ingredient,. Antacid Tablet Chemical Equation.
From www.numerade.com
One antacid tablet typically contains 600 mg of CaCO3. The calcium Antacid Tablet Chemical Equation Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. What is the formula for an antacid? In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. The base (the antacid) turns the. Antacid Tablet Chemical Equation.
From www.coursehero.com
[Solved] Weight of antacid tablet (Calcium 1.2579 g Carbonate Antacid Tablet Chemical Equation The base (the antacid) turns the acid primarily into salt and water. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. Commercial antacids consist of a number of ingredients, such as binders and flavorings, but the active ingredient is simply a basic salt such. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3},. Antacid Tablet Chemical Equation.
From talisman-intl.com
🎉 Antacid chemical reaction. Chemistry 104 Analysis of An Antacid Antacid Tablet Chemical Equation A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. The base (the antacid) turns the acid primarily into salt and water. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3},. Antacid Tablet Chemical Equation.
From www.numerade.com
⏩SOLVEDMagnesium hydroxide, Mg(OH)2, is commonly used as the active Antacid Tablet Chemical Equation What is the formula for an antacid? A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets. Antacid Tablet Chemical Equation.
From www.numerade.com
SOLVED Aluminum hydroxide in antacid tablets neutralize hydrochloric Antacid Tablet Chemical Equation \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. Commercial. Antacid Tablet Chemical Equation.
From www.coursehero.com
[Solved] I need help with my chemistry lab questions. 27.) Calculate Antacid Tablet Chemical Equation What is the formula for an antacid? A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day.. Antacid Tablet Chemical Equation.
From renatatara.blogspot.com
Antacid Tablet Chemical Formula An Antacid Tablet Contains Citric Antacid Tablet Chemical Equation What is the formula for an antacid? The base (the antacid) turns the acid primarily into salt and water. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to. Antacid Tablet Chemical Equation.
From www.chegg.com
Solved PART A To determine the salt formed during a Antacid Tablet Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. Commercial antacids consist of a number of ingredients, such as binders and flavorings, but the active ingredient is simply a basic salt such. The base (the antacid) turns the acid. Antacid Tablet Chemical Equation.
From www.behsscience.com
Untitled Document Antacid Tablet Chemical Equation The base (the antacid) turns the acid primarily into salt and water. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. What. Antacid Tablet Chemical Equation.
From www.vrogue.co
Class 7 Neutralisation Reaction Concept Formula Teach vrogue.co Antacid Tablet Chemical Equation \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. What is the formula for an antacid? In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost.. Antacid Tablet Chemical Equation.
From www.numerade.com
SOLVED The Neutralizing Capacity of Antacid Tablets Name Lab Antacid Tablet Chemical Equation The base (the antacid) turns the acid primarily into salt and water. Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. What is the formula for an antacid? A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2),. Antacid Tablet Chemical Equation.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Antacid Tablet Chemical Equation Although equation 2 shows that antacids can neutralize stomach acid, just how much antacid is needed to raise the ph of a full. \(\mathrm{mg}(\mathrm{oh})_{2}, \mathrm{nahco}_{3}, \mathrm{al}(\mathrm{oh})_{3}, \mathrm{caco}_{3}\) are the. The average therapeutic dose of antacid is 10 to 15 ml (1 tablespoon or one package content) of liquid or 1 to 2 tablets 3 to 4 times a day. Commercial. Antacid Tablet Chemical Equation.