What Does Heat Capacity Represent . The specific heat capacity of a material is a. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in.
from www.youtube.com
It plays a crucial role in understanding. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It is usually expressed as calories per degree in. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity of a material is a. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit.
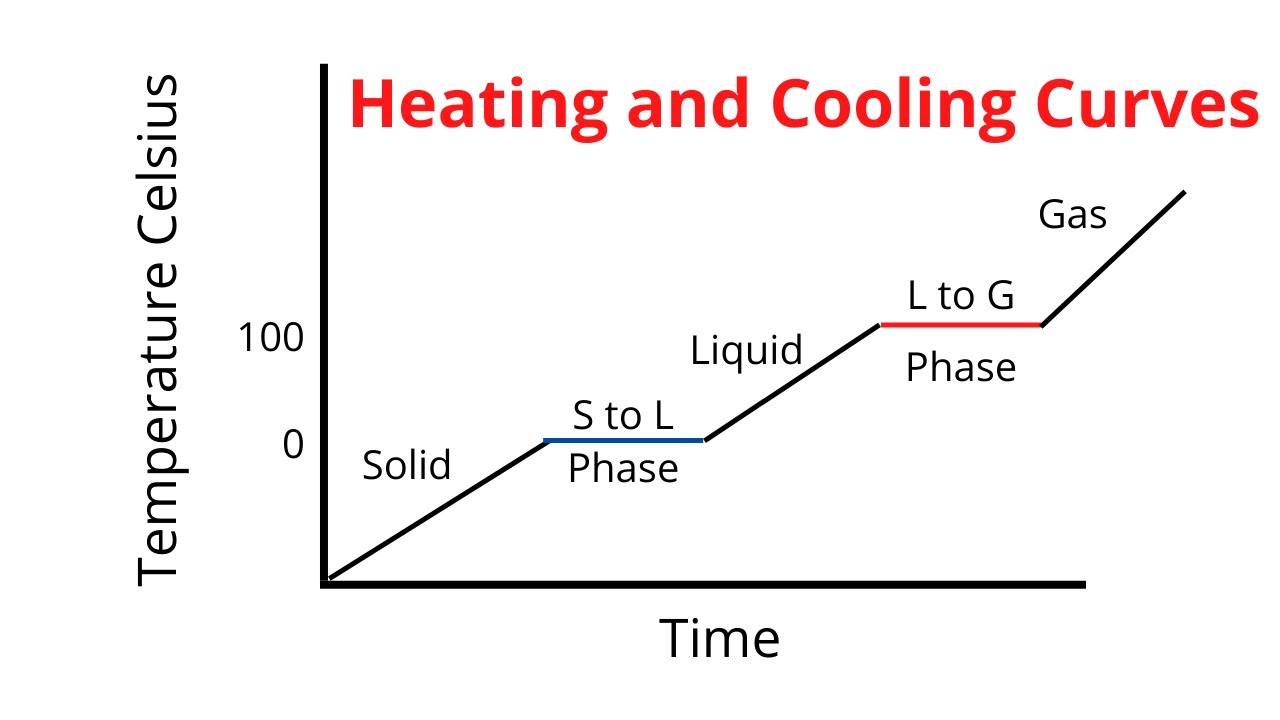
Heating and Cooling Curve / Introduction plus and Potential
What Does Heat Capacity Represent It plays a crucial role in understanding. It is usually expressed as calories per degree in. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. The specific heat capacity of a material is a. Heat capacity, ratio of heat absorbed by a material to the temperature change.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Does Heat Capacity Represent In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. It is. What Does Heat Capacity Represent.
From www.downwindkennels.com
Specific Heat Capacity What Does Heat Capacity Represent Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It. What Does Heat Capacity Represent.
From www.youtube.com
Finding Specific Heat Capacity IGCSE Physics YouTube What Does Heat Capacity Represent The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It plays a crucial role in understanding. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance. What Does Heat Capacity Represent.
From mytutorsource.hk
Learn About Specific Heat Capacity in Less Than 10 Minutes! What Does Heat Capacity Represent The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. The specific heat capacity of a material is a. It is usually expressed as calories per degree in. Specific heat is defined as the amount of heat required to raise the temperature of a unit. What Does Heat Capacity Represent.
From scienceinfo.com
Heat Capacity and Specific Heat What Does Heat Capacity Represent The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat capacity is the amount of heat energy required. What Does Heat Capacity Represent.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential What Does Heat Capacity Represent Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Specific heat capacity is the amount of heat energy required to raise the temperature of. What Does Heat Capacity Represent.
From slideplayer.com
Specific Heat Capacity ppt download What Does Heat Capacity Represent Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of. What Does Heat Capacity Represent.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Does Heat Capacity Represent Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is usually expressed as calories per degree in. The specific heat capacity of a material is a. It plays a crucial. What Does Heat Capacity Represent.
From sajeewasp.com
Physical chemistry Archives Sajeewa Pemasinghe What Does Heat Capacity Represent It plays a crucial role in understanding. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity. What Does Heat Capacity Represent.
From askfilo.com
4. Define heat capacity. What are Cp and Cv ? Show that CP −CV =R. A. T.. What Does Heat Capacity Represent The specific heat capacity of a material is a. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. In thermodynamics, the specific heat. What Does Heat Capacity Represent.
From www.coursehero.com
[Solved] What is the difference between the heat capacity and specific What Does Heat Capacity Represent It is usually expressed as calories per degree in. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity of a material is a. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat capacity is. What Does Heat Capacity Represent.
From www.youtube.com
Specific heat capacity YouTube What Does Heat Capacity Represent Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is usually expressed as calories per degree in. The specific heat capacity of a material is a. It plays a crucial. What Does Heat Capacity Represent.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Does Heat Capacity Represent Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is usually expressed as calories per degree in. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Heat capacity, ratio of heat. What Does Heat Capacity Represent.
From www.youtube.com
Heat Capacity YouTube What Does Heat Capacity Represent The specific heat capacity of a material is a. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Specific heat is defined as the amount of heat required to raise the. What Does Heat Capacity Represent.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Does Heat Capacity Represent Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. In thermodynamics, the specific heat capacity (symbol c) of a. What Does Heat Capacity Represent.
From physicscalculations.com
How to Calculate Specific Heat Capacity What Does Heat Capacity Represent The specific heat capacity of a material is a. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. In thermodynamics, the specific heat capacity (symbol c) of a substance is the. What Does Heat Capacity Represent.
From gambr.co
️Specific Heat Capacity Worksheet Gcse Free Download Gambr.co What Does Heat Capacity Represent Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. It is usually expressed as calories per degree in. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity of. What Does Heat Capacity Represent.
From www.nagwa.com
Question Video Finding the Specific Heat Capacity of a Substance given What Does Heat Capacity Represent The specific heat capacity of a material is a. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. Specific heat capacity is the amount of heat energy required to raise the temperature. What Does Heat Capacity Represent.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation What Does Heat Capacity Represent Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is usually expressed as calories per degree in. In thermodynamics, the specific heat capacity (symbol c) of a substance is the. What Does Heat Capacity Represent.
From byjus.com
The SI unit of specific heat capacity of a substance is What Does Heat Capacity Represent In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Heat capacity,. What Does Heat Capacity Represent.
From www.youtube.com
Specific Heat Equation Stated Clearly YouTube What Does Heat Capacity Represent In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs. What Does Heat Capacity Represent.
From novapublishers.com
Heat Capacity Theory and Measurement Nova Science Publishers What Does Heat Capacity Represent The specific heat capacity of a material is a. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity. What Does Heat Capacity Represent.
From www.mometrix.com
What is Specific Heat Capacity? (Video & FAQ) What Does Heat Capacity Represent Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. It is usually expressed as calories per degree in. It plays a crucial role in understanding. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit. What Does Heat Capacity Represent.
From studymind.co.uk
Heat Capacity Study Mind What Does Heat Capacity Represent It plays a crucial role in understanding. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. Specific heat capacity is the amount of heat energy required to raise the temperature of a. What Does Heat Capacity Represent.
From ar.inspiredpencil.com
Heat Capacity Of Water What Does Heat Capacity Represent Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of. What Does Heat Capacity Represent.
From www.tutorix.com
To determine specific heat capacity of given solid by method of mixtures What Does Heat Capacity Represent In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. Heat capacity, ratio of heat absorbed by a material to the temperature change. The specific heat capacity of a material is a. Specific. What Does Heat Capacity Represent.
From www.studypool.com
SOLUTION Specific heat capacity definition, importance, and formula What Does Heat Capacity Represent Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. It is usually expressed as calories per degree in. Specific heat is defined as the amount of heat required to raise the temperature of a unit. What Does Heat Capacity Represent.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity What Does Heat Capacity Represent In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. It is usually expressed as calories per degree in. Specific heat capacity is the amount of heat energy required to raise the temperature. What Does Heat Capacity Represent.
From ch301.cm.utexas.edu
heating curve What Does Heat Capacity Represent The specific heat capacity of a material is a. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. It is usually expressed as calories per degree in. Heat capacity, ratio of heat. What Does Heat Capacity Represent.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk What Does Heat Capacity Represent It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat. What Does Heat Capacity Represent.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources What Does Heat Capacity Represent The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is usually expressed as calories per degree in. Specific. What Does Heat Capacity Represent.
From www.toppr.com
() Give Its SI unit. (iii) How is the best capacity a body related to What Does Heat Capacity Represent The specific heat capacity of a material is a. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. What Does Heat Capacity Represent.
From calckit.io
CalcKit Specific Heat Capacity Converter What Does Heat Capacity Represent Heat capacity, ratio of heat absorbed by a material to the temperature change. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. Specific heat is defined as the amount of heat required. What Does Heat Capacity Represent.
From cuagodep.net
What Is The Molar Heat Capacity Of Water Unraveling Its Thermal Mysteries What Does Heat Capacity Represent The specific heat capacity of a material is a. Heat capacity, ratio of heat absorbed by a material to the temperature change. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit. Specific. What Does Heat Capacity Represent.
From slideplayer.com
Specific Heat Capacity ppt download What Does Heat Capacity Represent The specific heat capacity of a material is a. Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to. What Does Heat Capacity Represent.