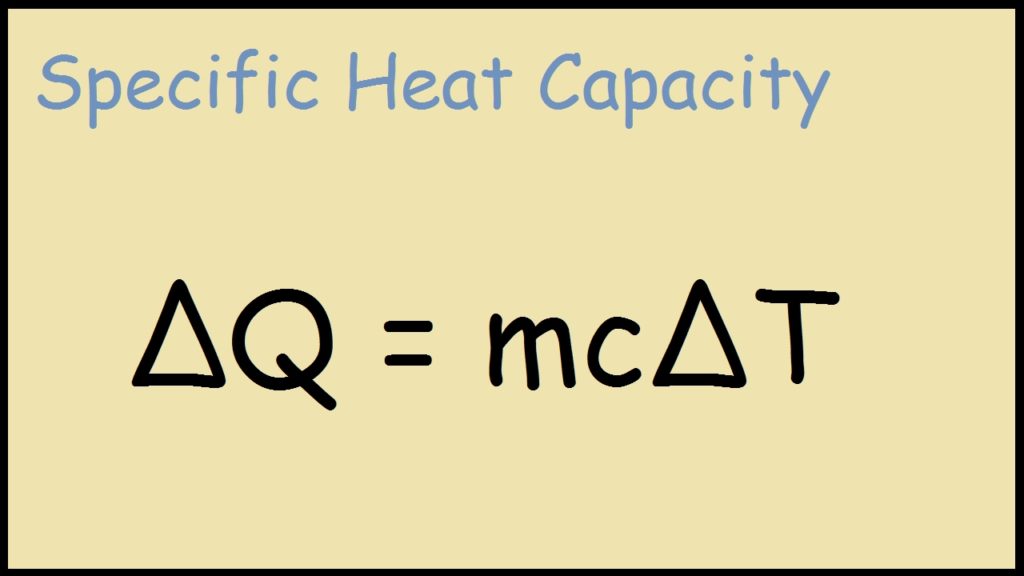Specific Heat Examples Biology . Water has the highest specific heat capacity of any liquids. It plays a crucial role in understanding how. It takes a lot of heat to increase the temperature of liquid water because some of the heat must be used to break hydrogen bonds between the molecules. Water has a high specific heat capacity. Water has the highest specific heat capacity of any liquid. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one. The units of specific heat are usually calories or joules per. High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. We define specific heat as the amount of heat one gram of a substance must absorb or lose to. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree celsius. Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin.
from www.toppr.com
Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Water has the highest specific heat capacity of any liquids. High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. It takes a lot of heat to increase the temperature of liquid water because some of the heat must be used to break hydrogen bonds between the molecules. The units of specific heat are usually calories or joules per. We define specific heat as the amount of heat one gram of a substance must absorb or lose to. Water has a high specific heat capacity. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree celsius. Water has the highest specific heat capacity of any liquid.
Specific Heat Formula Definition, Equations, Examples
Specific Heat Examples Biology We define specific heat as the amount of heat one gram of a substance must absorb or lose to. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. We define specific heat as the amount of heat one gram of a substance must absorb or lose to. It plays a crucial role in understanding how. The units of specific heat are usually calories or joules per. It takes a lot of heat to increase the temperature of liquid water because some of the heat must be used to break hydrogen bonds between the molecules. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree celsius. Water has the highest specific heat capacity of any liquid. Water has the highest specific heat capacity of any liquids. High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. Water has a high specific heat capacity. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one.
From www.slideshare.net
Specific Heat Specific Heat Examples Biology Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree celsius. High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. Specific heat, the quantity of heat required to raise the temperature of one. Specific Heat Examples Biology.
From slideplayer.com
Temperature, Heat, Specific Heat, & Heat Transfer ppt download Specific Heat Examples Biology Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one. It plays a crucial role in understanding how. High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. Specific heat capacity of water is the. Specific Heat Examples Biology.
From www.englishworksheet.my.id
Calculating Specific Heat Worksheet English Worksheet Specific Heat Examples Biology It plays a crucial role in understanding how. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Water has a high specific heat capacity.. Specific Heat Examples Biology.
From www.slideserve.com
PPT Chapter 2 The Energy Cycle PowerPoint Presentation, free download Specific Heat Examples Biology Water has the highest specific heat capacity of any liquids. It plays a crucial role in understanding how. Water has a high specific heat capacity. We define specific heat as the amount of heat one gram of a substance must absorb or lose to. Specific heat is defined as the amount of heat required to raise the temperature of a. Specific Heat Examples Biology.
From www.toppr.com
Specific Heat Formula Definition, Equations, Examples Specific Heat Examples Biology We define specific heat as the amount of heat one gram of a substance must absorb or lose to. High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. Water has the highest specific heat capacity of any liquids. Specific heat, the quantity of heat required to raise the. Specific Heat Examples Biology.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download Specific Heat Examples Biology Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Water has the highest specific heat capacity of any liquid. High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. The units of specific. Specific Heat Examples Biology.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID9277137 Specific Heat Examples Biology Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Water has the highest specific. Specific Heat Examples Biology.
From www.slideserve.com
PPT Enthalpy An introduction to Chemical Thermodynamics PowerPoint Specific Heat Examples Biology Water has the highest specific heat capacity of any liquids. It plays a crucial role in understanding how. Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by. Specific Heat Examples Biology.
From www.slideserve.com
PPT Science TAKS Facts PowerPoint Presentation, free download ID Specific Heat Examples Biology It plays a crucial role in understanding how. Water has the highest specific heat capacity of any liquid. It takes a lot of heat to increase the temperature of liquid water because some of the heat must be used to break hydrogen bonds between the molecules. Specific heat is defined as the amount of heat required to raise the temperature. Specific Heat Examples Biology.
From www.expii.com
High Specific Heat (Water) — Properties & Examples Expii Specific Heat Examples Biology Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin. We define specific heat as the amount of heat one gram of a substance must absorb or lose to. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance. Specific Heat Examples Biology.
From ar.inspiredpencil.com
Specific Heat Examples Everyday Life Specific Heat Examples Biology Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree celsius. Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin. High specific heat allows water to absorb large amounts of. Specific Heat Examples Biology.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 Specific Heat Examples Biology High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. We define specific heat as the amount of heat one gram of a substance must absorb or lose to. It takes a lot of heat to increase the temperature of liquid water because some of the heat must be. Specific Heat Examples Biology.
From classschoolwithstands.z21.web.core.windows.net
High Specific Heat In Water Specific Heat Examples Biology Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one. Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin. High specific heat allows water to absorb large amounts of heat without. Specific Heat Examples Biology.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems Specific Heat Examples Biology Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree celsius. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Water has a high specific heat capacity. Specific heat is defined as the. Specific Heat Examples Biology.
From education-portal.com
Latent Heat Definition, Formula & Examples Video & Lesson Transcript Specific Heat Examples Biology It plays a crucial role in understanding how. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Water has the highest specific heat capacity of any liquids. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Specific Heat Examples Biology.
From studylib.net
1.3 Specific Heat Capacity Specific Heat Examples Biology It plays a crucial role in understanding how. The units of specific heat are usually calories or joules per. High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change. Specific Heat Examples Biology.
From en.ppt-online.org
Class water online presentation Specific Heat Examples Biology Water has the highest specific heat capacity of any liquid. Water has the highest specific heat capacity of any liquids. The units of specific heat are usually calories or joules per. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat is defined. Specific Heat Examples Biology.
From dxoqtwvpm.blob.core.windows.net
What Is Heat Capacity In Materials at James Eng blog Specific Heat Examples Biology It plays a crucial role in understanding how. Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin. High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. Specific heat is defined as the amount. Specific Heat Examples Biology.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download Specific Heat Examples Biology We define specific heat as the amount of heat one gram of a substance must absorb or lose to. The units of specific heat are usually calories or joules per. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Specific heat capacity of water is the amount of. Specific Heat Examples Biology.
From www.youtube.com
Specific Heat Capacity Short Example Solving for Mass YouTube Specific Heat Examples Biology Water has the highest specific heat capacity of any liquids. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree celsius. Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin.. Specific Heat Examples Biology.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID2124311 Specific Heat Examples Biology Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin. High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. Specific heat, the quantity of heat required to raise the temperature of one gram of. Specific Heat Examples Biology.
From www.youtube.com
Specific heat capacity YouTube Specific Heat Examples Biology Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one. High specific heat allows water to absorb large amounts of heat without. Specific Heat Examples Biology.
From www.saralmind.com
Heat Notes, Videos, QA and Tests Class 10>Science>Heat SaralMind Specific Heat Examples Biology We define specific heat as the amount of heat one gram of a substance must absorb or lose to. Water has the highest specific heat capacity of any liquids. It plays a crucial role in understanding how. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by. Specific Heat Examples Biology.
From study.com
Heat of Fusion Definition, Equation & Examples Video & Lesson Specific Heat Examples Biology Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one. We. Specific Heat Examples Biology.
From www.youtube.com
Specific heat capacity (with problems and solutions) YouTube Specific Heat Examples Biology The units of specific heat are usually calories or joules per. Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin. Water has the highest specific heat capacity of any liquids. Specific heat is defined as the amount of heat one gram of a substance must. Specific Heat Examples Biology.
From www.slideserve.com
PPT Thermodynamics and Specific Heat PowerPoint Presentation, free Specific Heat Examples Biology It plays a crucial role in understanding how. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Water has the highest specific heat capacity of any liquids. It takes a lot of heat to increase the temperature of liquid water because some of the heat must be used. Specific Heat Examples Biology.
From ar.inspiredpencil.com
Specific Heat Of Water Jkg C Specific Heat Examples Biology It plays a crucial role in understanding how. Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of 1kg of water by 1 kelvin. We define specific heat as the amount of heat one gram of a substance must absorb or lose to. High specific heat allows water to absorb large amounts. Specific Heat Examples Biology.
From www.slideserve.com
PPT Laws of Thermodynamics PowerPoint Presentation, free download Specific Heat Examples Biology High specific heat allows water to absorb large amounts of heat without significantly changing its temperature, which is essential for regulating. Water has a high specific heat capacity. It takes a lot of heat to increase the temperature of liquid water because some of the heat must be used to break hydrogen bonds between the molecules. Water has the highest. Specific Heat Examples Biology.
From cuagodep.net
What Is The Molar Heat Capacity Of Water Unraveling Its Thermal Mysteries Specific Heat Examples Biology The units of specific heat are usually calories or joules per. It plays a crucial role in understanding how. Water has the highest specific heat capacity of any liquid. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Water has the highest specific heat capacity of any liquids.. Specific Heat Examples Biology.
From www.pinterest.com
Energy, Solar Energy, Definition Of Science, Physics Concepts Specific Heat Examples Biology Water has the highest specific heat capacity of any liquid. Water has a high specific heat capacity. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat capacity of water is the amount of heat energy (j) needed to raise the temperature of. Specific Heat Examples Biology.
From www.slideserve.com
PPT Heat Capacity and Specific Heat Capacity PowerPoint Presentation Specific Heat Examples Biology It takes a lot of heat to increase the temperature of liquid water because some of the heat must be used to break hydrogen bonds between the molecules. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree celsius. Water has the highest specific heat. Specific Heat Examples Biology.
From heatklp.blogspot.com
11+ Why Is Heat Of Vaporization Important To Life On Earth Article Specific Heat Examples Biology It takes a lot of heat to increase the temperature of liquid water because some of the heat must be used to break hydrogen bonds between the molecules. Water has the highest specific heat capacity of any liquid. We define specific heat as the amount of heat one gram of a substance must absorb or lose to. Specific heat is. Specific Heat Examples Biology.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Specific Heat Examples Biology We define specific heat as the amount of heat one gram of a substance must absorb or lose to. Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Water has the highest specific heat capacity of any liquids. Water has a high specific heat capacity. Specific heat is. Specific Heat Examples Biology.
From studylib.net
Heat Equation Specific Heat Examples Biology Water has the highest specific heat capacity of any liquid. It takes a lot of heat to increase the temperature of liquid water because some of the heat must be used to break hydrogen bonds between the molecules. We define specific heat as the amount of heat one gram of a substance must absorb or lose to. Specific heat is. Specific Heat Examples Biology.
From www.slideserve.com
PPT Molecules of Life PowerPoint Presentation, free download ID1321531 Specific Heat Examples Biology Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree celsius. High specific heat allows water to absorb large amounts. Specific Heat Examples Biology.