The Solution Is Dilution Lab Answers . Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Of course, the resulting solution is thoroughly mixed so as to. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Explain how concentrations can be changed in the lab. You dilute the solution to 600 ml. Understand how stock solutions are used in the laboratory. Use serial dilution to make a 2 mm solution from a 2 m stock solution. To dilute a solution means to add more solvent without the addition of more solute.
from www.chegg.com
To dilute a solution means to add more solvent without the addition of more solute. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Use serial dilution to make a 2 mm solution from a 2 m stock solution. You dilute the solution to 600 ml. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Of course, the resulting solution is thoroughly mixed so as to. Understand how stock solutions are used in the laboratory. Explain how concentrations can be changed in the lab.
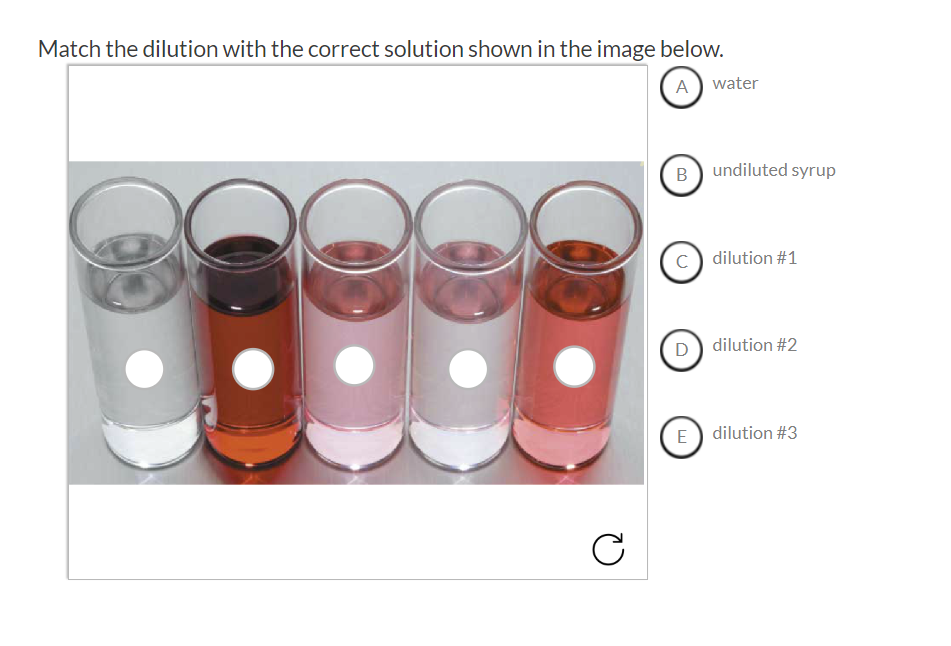
Solved Match the dilution with the correct solution shown in
The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Use serial dilution to make a 2 mm solution from a 2 m stock solution. To dilute a solution means to add more solvent without the addition of more solute. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? You dilute the solution to 600 ml. Explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Of course, the resulting solution is thoroughly mixed so as to.
From www.chegg.com
Solved PreLab Assignment Experiment 7 The Solution is The Solution Is Dilution Lab Answers Explain how concentrations can be changed in the lab. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Use serial dilution to make a 2 mm solution from a 2 m stock solution. You are given a 1 m nacl solution and need a 10. The Solution Is Dilution Lab Answers.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory The Solution Is Dilution Lab Answers Of course, the resulting solution is thoroughly mixed so as to. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. To dilute a solution means to add more solvent without the addition of more solute. Explain how concentrations can be changed in the lab. You are given a 1 m nacl solution. The Solution Is Dilution Lab Answers.
From classfullkay.z13.web.core.windows.net
Molarity By Dilution Worksheets Answers The Solution Is Dilution Lab Answers To dilute a solution means to add more solvent without the addition of more solute. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. Use serial dilution. The Solution Is Dilution Lab Answers.
From klafwpnrs.blob.core.windows.net
Dilution Formula Explanation at John Schell blog The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Of course, the resulting solution is thoroughly mixed so as to. Understand how stock solutions are used in the. The Solution Is Dilution Lab Answers.
From www.chegg.com
Solved Question During The First Week Of This Project, Y... The Solution Is Dilution Lab Answers Explain how concentrations can be changed in the lab. Of course, the resulting solution is thoroughly mixed so as to. Understand how stock solutions are used in the laboratory. You are given a 1 m nacl solution and need a 10 mm nacl solution for. To dilute a solution means to add more solvent without the addition of more solute.. The Solution Is Dilution Lab Answers.
From www.chegg.com
Solved Serial dilution is a common technique used in The Solution Is Dilution Lab Answers To dilute a solution means to add more solvent without the addition of more solute. Explain how concentrations can be changed in the lab. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. Use serial dilution to make a 2 mm solution from a 2 m stock solution. You dilute the solution. The Solution Is Dilution Lab Answers.
From ar.inspiredpencil.com
Serial Dilution Diagram The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. Of course, the resulting solution is thoroughly mixed so as to. Understand how stock solutions are used in the laboratory. Explain how concentrations can be changed in the lab. To dilute a solution means to add more solvent without the addition of more. The Solution Is Dilution Lab Answers.
From studylib.net
Lab The Dilution Solution The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. You dilute the solution to 600 ml. Understand how stock solutions are used in the laboratory. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Use serial dilution to make a 2 mm solution from a. The Solution Is Dilution Lab Answers.
From www.chegg.com
Solved Lab 5 Practice Dilution Problems Q1 You have a The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. To dilute a solution means to add more solvent without the addition of more solute. Understand how stock solutions are used in the laboratory. Explain how concentrations can be changed in the lab. Use serial dilution to make a 2 mm solution from. The Solution Is Dilution Lab Answers.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts The Solution Is Dilution Lab Answers What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? You are given a 1 m nacl solution and need a 10 mm nacl solution for. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. Study with quizlet. The Solution Is Dilution Lab Answers.
From www.chegg.com
Solved Dilution diagrams can be very helpful in organizing The Solution Is Dilution Lab Answers You dilute the solution to 600 ml. Understand how stock solutions are used in the laboratory. To dilute a solution means to add more solvent without the addition of more solute. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Explain how concentrations can be changed in the lab.. The Solution Is Dilution Lab Answers.
From exytxbygt.blob.core.windows.net
Dilution Definition Literature at Mark Foster blog The Solution Is Dilution Lab Answers Understand how stock solutions are used in the laboratory. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. Explain how concentrations can be changed in the lab. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Use serial dilution to make. The Solution Is Dilution Lab Answers.
From www.scribd.com
The Solution Is Dilution! Lab Investigation PDF Concentration The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Of course, the resulting solution is thoroughly mixed so as to. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. To dilute a solution means to add more solvent without the addition. The Solution Is Dilution Lab Answers.
From www.chegg.com
Solved Prelab Assignment The Solution is Dilution The Solution Is Dilution Lab Answers Understand how stock solutions are used in the laboratory. Explain how concentrations can be changed in the lab. Use serial dilution to make a 2 mm solution from a 2 m stock solution. You dilute the solution to 600 ml. To dilute a solution means to add more solvent without the addition of more solute. Study with quizlet and memorize. The Solution Is Dilution Lab Answers.
From www.chegg.com
Solved Match the dilution with the correct solution shown in The Solution Is Dilution Lab Answers You are given a 1 m nacl solution and need a 10 mm nacl solution for. You dilute the solution to 600 ml. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the.. The Solution Is Dilution Lab Answers.
From www.scribd.com
78 Dilution Lab Experiment Solution Free 30day Trial Scribd The Solution Is Dilution Lab Answers You dilute the solution to 600 ml. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. To dilute a solution means to add more solvent without the addition of more solute. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Use. The Solution Is Dilution Lab Answers.
From www.docsity.com
Making Dilutions Worksheet Answers Key Docsity The Solution Is Dilution Lab Answers You are given a 1 m nacl solution and need a 10 mm nacl solution for. Understand how stock solutions are used in the laboratory. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. You dilute the solution to 600 ml. What is the required dilution factor for each step, and how. The Solution Is Dilution Lab Answers.
From www.chegg.com
Solved You are making 2fold serial dilutions (12). Your The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. What is the required dilution factor for each step, and how much of the. The Solution Is Dilution Lab Answers.
From studylib.net
Dilution The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Explain how concentrations can be changed in the lab. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Of course, the resulting solution is thoroughly mixed so as to. Understand how stock solutions. The Solution Is Dilution Lab Answers.
From www.studypool.com
SOLUTION Answer key lab activity kool aid concentration pdfdrive The Solution Is Dilution Lab Answers To dilute a solution means to add more solvent without the addition of more solute. Understand how stock solutions are used in the laboratory. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from. The Solution Is Dilution Lab Answers.
From www.numerade.com
Help, I'm not sure if I'm doing this dilution problem correctly. 21 The Solution Is Dilution Lab Answers To dilute a solution means to add more solvent without the addition of more solute. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Explain how concentrations can be changed in the lab. You dilute the solution to 600 ml. Of course, the resulting solution is thoroughly mixed so as to. Study with. The Solution Is Dilution Lab Answers.
From www.studocu.com
Solution and Dilution Lab101 S24 Solution or Dilution? Materials The Solution Is Dilution Lab Answers Of course, the resulting solution is thoroughly mixed so as to. Use serial dilution to make a 2 mm solution from a 2 m stock solution. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? You dilute the solution to 600 ml. Study with quizlet. The Solution Is Dilution Lab Answers.
From dxopazssz.blob.core.windows.net
Dilution Calculator From Mass at Blanca Norton blog The Solution Is Dilution Lab Answers You dilute the solution to 600 ml. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Use serial dilution to make a 2 mm solution from a 2 m stock solution. Explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. What is the required. The Solution Is Dilution Lab Answers.
From www.academia.edu
(PDF) Experiment 16 The Solution is Dilution Lirim Sopaj The Solution Is Dilution Lab Answers Explain how concentrations can be changed in the lab. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Understand how stock solutions are used in. The Solution Is Dilution Lab Answers.
From www.chegg.com
Solved In the image above, the final dilution is 1 You The Solution Is Dilution Lab Answers Of course, the resulting solution is thoroughly mixed so as to. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Use serial dilution to make a 2 mm solution from a 2 m stock solution. You are given a 1 m nacl solution and need. The Solution Is Dilution Lab Answers.
From studylib.net
Chem 1020 Molarity worksheet, version 2 1. What is the molarity of a The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Explain how concentrations can be changed in the lab. You dilute the solution to 600 ml. Of course, the resulting solution is thoroughly. The Solution Is Dilution Lab Answers.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. You are given a 1 m nacl solution and need a 10 mm nacl solution for. You dilute the solution to 600 ml. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the.. The Solution Is Dilution Lab Answers.
From studylib.net
Well, What Will We Drink? Ppm, ppb, and Serial Dilution Lab The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. You dilute the solution to 600 ml. What is the required dilution factor for each step, and how much of the original solution. The Solution Is Dilution Lab Answers.
From www.pinterest.se
Dilution when solvent is added to dilute a solution, the number of The Solution Is Dilution Lab Answers Use serial dilution to make a 2 mm solution from a 2 m stock solution. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. You. The Solution Is Dilution Lab Answers.
From studylib.net
Lab Is Dilution the Solution The Solution Is Dilution Lab Answers Use serial dilution to make a 2 mm solution from a 2 m stock solution. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. To dilute a solution means to add more solvent without the addition of more solute. Understand how stock solutions are used in the laboratory. Study. The Solution Is Dilution Lab Answers.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory The Solution Is Dilution Lab Answers Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. Use serial dilution to make a 2 mm solution from a 2 m stock solution. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet solution (from the. Explain how concentrations can be changed in the. The Solution Is Dilution Lab Answers.
From mungfali.com
10 Fold Serial Dilution The Solution Is Dilution Lab Answers You dilute the solution to 600 ml. Explain how concentrations can be changed in the lab. Of course, the resulting solution is thoroughly mixed so as to. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Study with quizlet and memorize flashcards containing terms like data table 1 molarity of concentrated crystal violet. The Solution Is Dilution Lab Answers.
From www.judithcahen.net
Molarity And Dilution Phet Lab Answer Key » Judithcahen Answer Key For The Solution Is Dilution Lab Answers To dilute a solution means to add more solvent without the addition of more solute. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Of course, the resulting solution is thoroughly mixed so as to. Understand how stock solutions are used in the laboratory. Use serial dilution to make a 2 mm solution. The Solution Is Dilution Lab Answers.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query The Solution Is Dilution Lab Answers What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Understand how stock solutions are used in the laboratory. Explain how concentrations can be changed in the lab. You dilute the solution to 600 ml. Use serial dilution to make a 2 mm solution from a. The Solution Is Dilution Lab Answers.
From loegnwkyw.blob.core.windows.net
Serial Dilution Equation Examples at Keith Rush blog The Solution Is Dilution Lab Answers Of course, the resulting solution is thoroughly mixed so as to. What is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? To dilute a solution means to add more solvent without the addition of more solute. Explain how concentrations can be changed in the lab. You. The Solution Is Dilution Lab Answers.