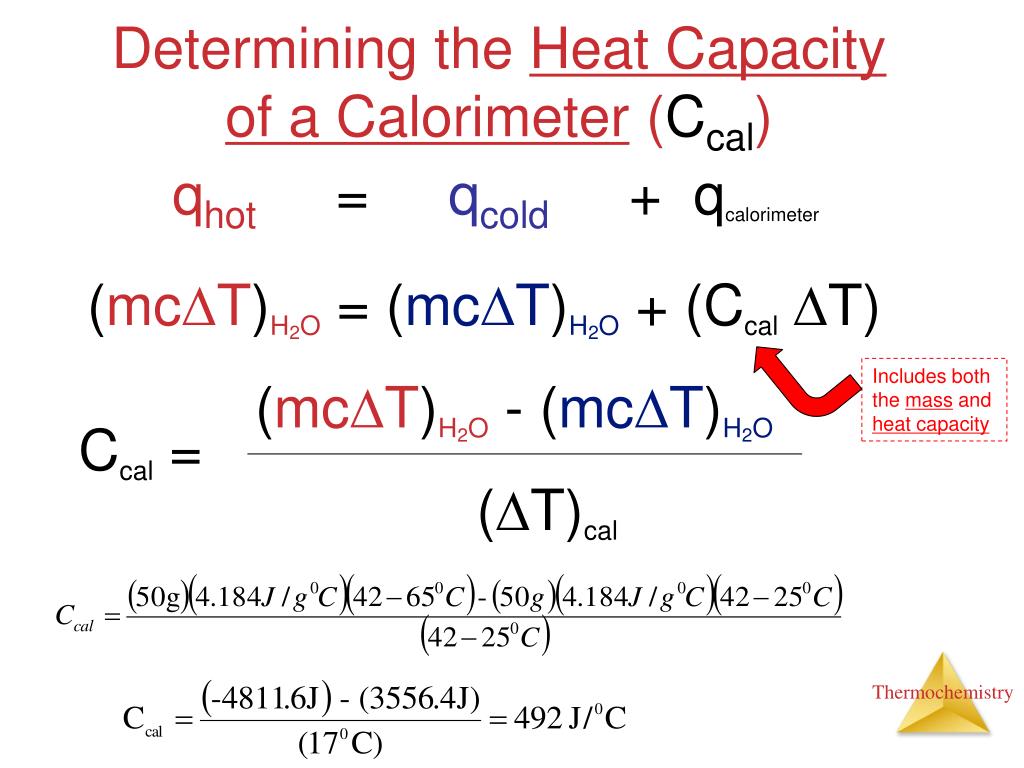
Why Is It Important To Determine The Heat Capacity Of The Calorimeter . a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a calorimeter is a device used to determine heat flow during a chemical or physical change. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the temperature change produced by the known reaction is used to determine the heat capacity of the.
from www.slideserve.com
a calorimeter is a device used to determine heat flow during a chemical or physical change. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. the temperature change produced by the known reaction is used to determine the heat capacity of the. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a.
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download
Why Is It Important To Determine The Heat Capacity Of The Calorimeter bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the temperature change produced by the known reaction is used to determine the heat capacity of the. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. a calorimeter is a device used to determine heat flow during a chemical or physical change. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results.
From cewwmzhd.blob.core.windows.net
What Is The Heat Capacity Of The Calorimeter Due To at Peter Brunton blog Why Is It Important To Determine The Heat Capacity Of The Calorimeter a calorimeter is a device used to determine heat flow during a chemical or physical change. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. the temperature change produced by the known reaction is used to. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Why Is It Important To Determine The Heat Capacity Of The Calorimeter the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a container that prevents heat transfer in or out is called a calorimeter, and the use of a. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.numerade.com
SOLVED1. (S points) What is "heal capacity' 2 (5 points) Why do You Why Is It Important To Determine The Heat Capacity Of The Calorimeter bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the temperature change produced by the known reaction is used to determine the heat capacity of the. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, or constant Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. the temperature change produced by the known reaction is used to determine the heat capacity of the. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the temperature change produced by the known reaction is used to determine the heat capacity of. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Why Is It Important To Determine The Heat Capacity Of The Calorimeter a calorimeter is a device used to determine heat flow during a chemical or physical change. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results.. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From zuoti.pro
b. In a calorimetric exp periment, why is it important to determine the Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the heat. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. the temperature. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Why Is It Important To Determine The Heat Capacity Of The Calorimeter the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. a calorimeter is a device used to determine heat flow during a chemical or physical change. bomb calorimeters require calibration. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. a container that prevents heat transfer in or out. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From chemistrytalk.org
Calorimetry ChemTalk Why Is It Important To Determine The Heat Capacity Of The Calorimeter the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Why Is It Important To Determine The Heat Capacity Of The Calorimeter the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. a calorimeter is a device used to determine heat flow during a chemical or physical change. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. the temperature change produced. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From punchlistzero.com
Is Heat a State Function? Punchlist Zero Why Is It Important To Determine The Heat Capacity Of The Calorimeter a calorimeter is a device used to determine heat flow during a chemical or physical change. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.chegg.com
Solved To determine the heat capacity of a calorimeter you Why Is It Important To Determine The Heat Capacity Of The Calorimeter the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a container. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature change produced by the known reaction is used to determine the heat capacity of the. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a container that prevents heat transfer in. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Why Is It Important To Determine The Heat Capacity Of The Calorimeter bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the temperature change produced by the known reaction is used to determine the heat capacity of the. the temperature increase is measured and, along. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.youtube.com
050 Calorimetry YouTube Why Is It Important To Determine The Heat Capacity Of The Calorimeter the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. the temperature change produced by the known reaction is used to determine the heat capacity of the. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. . Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature change produced by the known reaction is used to determine the heat capacity of the. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From saylordotorg.github.io
Calorimetry Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature change produced by the known reaction is used to determine the heat capacity of the. a calorimeter is a device used to determine heat flow during a chemical or physical change. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Why Is It Important To Determine The Heat Capacity Of The Calorimeter bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a calorimeter is a device used to determine heat flow during a chemical or physical change. the temperature change produced by the known reaction is used to determine the heat capacity of the. the combustion of 1 mole of glucose. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Why Is It Important To Determine The Heat Capacity Of The Calorimeter bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. bomb. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.youtube.com
Principle of Calorimetry YouTube Why Is It Important To Determine The Heat Capacity Of The Calorimeter a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. a calorimeter is a device used to determine heat flow during a chemical or physical change. the. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From slidetodoc.com
Determine the heat capacity in caloriesC of a Why Is It Important To Determine The Heat Capacity Of The Calorimeter the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. bomb calorimeters require calibration to determine the heat. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.numerade.com
SOLVED Why is it important to determine the heat capacity of the Why Is It Important To Determine The Heat Capacity Of The Calorimeter the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. bomb calorimeters require calibration to determine the heat capacity. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature change produced by the known reaction is used to determine the heat capacity of the. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.sarthaks.com
Figure shows an electrical calorimeter to determine specific heat Why Is It Important To Determine The Heat Capacity Of The Calorimeter a calorimeter is a device used to determine heat flow during a chemical or physical change. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. the temperature increase is measured and, along with the known heat capacity of the calorimeter,. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From studylib.net
TAP 607 3 Measurement of specific heat capacities Why Is It Important To Determine The Heat Capacity Of The Calorimeter the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. the temperature change produced by the known reaction is used to determine the heat capacity of the. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. . Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From quizunderfires.z21.web.core.windows.net
How To Use A Calorimeter Why Is It Important To Determine The Heat Capacity Of The Calorimeter bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. the temperature change produced by the known reaction is used to determine the heat capacity. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Why Is It Important To Determine The Heat Capacity Of The Calorimeter a calorimeter is a device used to determine heat flow during a chemical or physical change. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. the temperature change produced. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature change produced by the known reaction is used to determine the heat capacity of the. a calorimeter is a device used to determine heat flow during a chemical or physical change. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a container that prevents heat transfer in. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From library.achievingthedream.org
Calorimetry Introductory Chemistry Lecture & Lab Why Is It Important To Determine The Heat Capacity Of The Calorimeter the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow Why Is It Important To Determine The Heat Capacity Of The Calorimeter bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. the heat capacity (c) of a body of matter is the quantity of heat (q). Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Why Is It Important To Determine The Heat Capacity Of The Calorimeter bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the reaction. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results.. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Why Is It Important To Determine The Heat Capacity Of The Calorimeter the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. the temperature change produced by the known reaction is used to determine the heat capacity of the. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. . Why Is It Important To Determine The Heat Capacity Of The Calorimeter.
From www.numerade.com
SOLVEDUse the References to access important values if needed for this Why Is It Important To Determine The Heat Capacity Of The Calorimeter bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. a calorimeter is a. Why Is It Important To Determine The Heat Capacity Of The Calorimeter.