Lead Chloride Is Highly Unstable Towards Heat . • lead is known not to. +2 oxidation of pb is more stable due to inert pair effect. Lead(ii) chloride does not react with `cl_(2)` to give `pbcl_(4)`. Lead is known not to form an iodide pbl 4. Lead (iv) chloride is highly unstable towards heat. • lead (iv) chloride is highly unstable towards heat. • lead (ii) chloride reacts with cl 2 to give pbcl 4. • lead (iv) chloride is highly unstable towards heat. (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. • lead is known not to form an iodide, p bi 4. (b) lead(iv) chloride is highly unstable towards heat. Lead(iv) chloride is highly unstable towards heat. Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. On moving down group iv, the higher oxidation state becomes unstable because of the inert. (a) lead belongs to group 14 of the.
from classnotes.guru
• lead (ii) chloride reacts with cl 2 to give pbcl 4. (a) lead belongs to group 14 of the. • lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. • lead is known not to form an iodide, p bi 4. Lead(iv) chloride is highly unstable towards heat. Lead(ii) chloride does not react with `cl_(2)` to give `pbcl_(4)`. Lead (ii) chloride reacts with cl 2 to give pbcl 4. Lead is known not to form an iodide pbl 4. On moving down group iv, the higher oxidation state becomes unstable because of the inert.
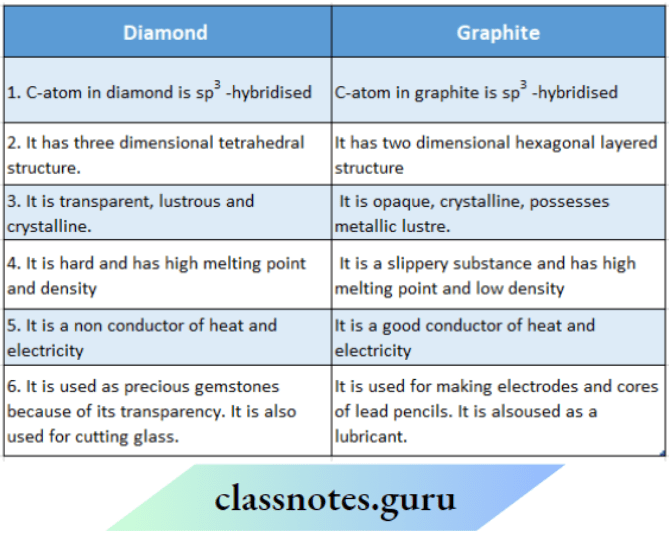
NCERT Class 11 Chemistry Chapter 11 Some P Block Elements Long Question
Lead Chloride Is Highly Unstable Towards Heat Lead (iv) chloride is highly unstable towards heat. (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. • lead is known not to. (a) lead belongs to group 14 of the. P b c l 2 + c l 2 → p b c l 4. Lead (ii) chloride reacts with cl 2 to give pbcl 4. Lead (iv) chloride is highly unstable towards heat. On moving down group iv, the higher oxidation state becomes unstable because of the inert. Lead(ii) chloride does not react with `cl_(2)` to give `pbcl_(4)`. Lead (iv) chloride is highly unstable towards heat. • lead (iv) chloride is highly unstable towards heat. Lead is known not to form an iodide pbl 4. • lead is known not to form an iodide, p bi 4. +2 oxidation of pb is more stable due to inert pair effect. Lead (iv) chloride is highly unstable towards heat.
From www.indiamart.com
Lead Chloride powder, For Industrial, Packaging Type 50 Kg Hdpe Bag at Lead Chloride Is Highly Unstable Towards Heat • lead is known not to form an iodide, p bi 4. Lead (iv) chloride is highly unstable towards heat. (b) lead(iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. Lead (ii) chloride reacts with cl 2 to give pbcl 4. P b c l 2 + c l 2 → p b c. Lead Chloride Is Highly Unstable Towards Heat.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Is Highly Unstable Towards Heat Lead(iv) chloride is highly unstable towards heat. (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. Lead (iv) chloride is highly unstable towards heat. • lead is known not to form an iodide, p bi 4. (b) lead(iv) chloride is highly unstable towards heat. Lead (iv). Lead Chloride Is Highly Unstable Towards Heat.
From www.numerade.com
When lead chloride (PbClz) is placed in otherwise pure water; enough Lead Chloride Is Highly Unstable Towards Heat Lead (iv) chloride is highly unstable towards heat. (b) lead(iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. • lead is known not to. On moving down group iv, the higher oxidation state becomes unstable because of the inert. Lead is known not to form an iodide pbl 4. • lead (iv) chloride is. Lead Chloride Is Highly Unstable Towards Heat.
From www.indiamart.com
Lead Chloride Powder at best price in Mumbai by Krishna Metal Refinery Lead Chloride Is Highly Unstable Towards Heat (b) lead(iv) chloride is highly unstable towards heat. Lead(ii) chloride does not react with `cl_(2)` to give `pbcl_(4)`. Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. On moving. Lead Chloride Is Highly Unstable Towards Heat.
From www.solutionspile.com
[Solved] The equation for the dissolving of lead (II) Lead Chloride Is Highly Unstable Towards Heat Lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. • lead (iv) chloride is highly unstable towards heat. (b) lead(iv) chloride is highly unstable towards heat. • lead (ii) chloride reacts with cl 2 to give pbcl 4. (a) lead belongs to group 14 of the. Lead (iv) chloride is highly unstable towards. Lead Chloride Is Highly Unstable Towards Heat.
From www.youtube.com
Double displacement NaCl + Pb(NO3)2 Sodium chloride + Lead(ii Lead Chloride Is Highly Unstable Towards Heat Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. Lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. (a) lead belongs to group 14 of the. +2 oxidation of pb is more stable due to inert pair effect. • lead is known not to. Lead. Lead Chloride Is Highly Unstable Towards Heat.
From www.teachoo.com
Case Based MCQ The following diagram displays a chemical reaction Lead Chloride Is Highly Unstable Towards Heat (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. • lead (iv) chloride is highly unstable towards heat. • lead is known not to. +2 oxidation of pb is more stable due to inert pair effect. • lead (iv) chloride is highly unstable towards heat. P. Lead Chloride Is Highly Unstable Towards Heat.
From www.mittalpigments.net
Manufacturer, Exporter & Supplier of Grey Lead Oxide in Rajasthan India Lead Chloride Is Highly Unstable Towards Heat • lead (iv) chloride is highly unstable towards heat. On moving down group iv, the higher oxidation state becomes unstable because of the inert. +2 oxidation of pb is more stable due to inert pair effect. • lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. Lead is known not to form an. Lead Chloride Is Highly Unstable Towards Heat.
From www.indiamart.com
Lead Chloride Powder, 98, Loose at Rs 420/kg in Valsad ID 27292324712 Lead Chloride Is Highly Unstable Towards Heat • lead (iv) chloride is highly unstable towards heat. Lead(iv) chloride is highly unstable towards heat. Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. • lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. (b) lead(iv) chloride is highly unstable towards heat. (ii) lead. Lead Chloride Is Highly Unstable Towards Heat.
From classnotes.guru
NCERT Class 11 Chemistry Chapter 11 Some P Block Elements Long Question Lead Chloride Is Highly Unstable Towards Heat • lead is known not to form an iodide, p bi 4. P b c l 2 + c l 2 → p b c l 4. • lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. Lead(iv) chloride is highly unstable towards heat. (ii) lead tetrachloride ( p b c l 4). Lead Chloride Is Highly Unstable Towards Heat.
From www.gauthmath.com
Drag each statement to the correct location on the chart. Classify the Lead Chloride Is Highly Unstable Towards Heat On moving down group iv, the higher oxidation state becomes unstable because of the inert. Lead (iv) chloride is highly unstable towards heat. • lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. Lead(ii) chloride does not react with `cl_(2)` to give `pbcl_(4)`. Answers (1) on moving down the group the +4 oxidation. Lead Chloride Is Highly Unstable Towards Heat.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Is Highly Unstable Towards Heat • lead is known not to form an iodide, p bi 4. • lead is known not to. Lead (iv) chloride is highly unstable towards heat. P b c l 2 + c l 2 → p b c l 4. • lead (iv) chloride is highly unstable towards heat. • lead (ii) chloride reacts with cl 2 to give. Lead Chloride Is Highly Unstable Towards Heat.
From encyclopedia.pub
Compounds of Lead Encyclopedia MDPI Lead Chloride Is Highly Unstable Towards Heat • lead is known not to form an iodide, p bi 4. • lead (ii) chloride reacts with cl 2 to give pbcl 4. Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. On moving down group iv, the higher oxidation state becomes unstable because of the inert. Lead is known not. Lead Chloride Is Highly Unstable Towards Heat.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Lead Chloride Is Highly Unstable Towards Heat • lead (iv) chloride is highly unstable towards heat. P b c l 2 + c l 2 → p b c l 4. Lead (ii) chloride reacts with cl 2 to give pbcl 4. Lead(iv) chloride is highly unstable towards heat. Lead(ii) chloride does not react with `cl_(2)` to give `pbcl_(4)`. On moving down group iv, the higher oxidation. Lead Chloride Is Highly Unstable Towards Heat.
From www.coursehero.com
[Solved] why does aluminum react with copper sulfate + sodium chloride Lead Chloride Is Highly Unstable Towards Heat (a) lead belongs to group 14 of the. • lead is known not to form an iodide, p bi 4. Lead (ii) chloride reacts with cl 2 to give pbcl 4. Lead(iv) chloride is highly unstable towards heat. • lead (ii) chloride reacts with cl 2 to give pbcl 4. On moving down group iv, the higher oxidation state becomes. Lead Chloride Is Highly Unstable Towards Heat.
From www.chegg.com
Solved Compare the solubility of lead chloride in each of Lead Chloride Is Highly Unstable Towards Heat • lead is known not to form an iodide, p bi 4. Lead (ii) chloride reacts with cl 2 to give pbcl 4. • lead (iv) chloride is highly unstable towards heat. • lead (ii) chloride reacts with cl 2 to give pbcl 4. Lead (iv) chloride is highly unstable towards heat. +2 oxidation of pb is more stable due. Lead Chloride Is Highly Unstable Towards Heat.
From www.toppr.com
Br 77. Al in Br The reaction of 4bromobenzyl chloride with NaCN in Lead Chloride Is Highly Unstable Towards Heat On moving down group iv, the higher oxidation state becomes unstable because of the inert. P b c l 2 + c l 2 → p b c l 4. Lead (ii) chloride reacts with cl 2 to give pbcl 4. • lead is known not to. Lead (iv) chloride is highly unstable towards heat. • lead (iv) chloride is. Lead Chloride Is Highly Unstable Towards Heat.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Is Highly Unstable Towards Heat Lead (iv) chloride is highly unstable towards heat. Lead (ii) chloride reacts with cl 2 to give pbcl 4. Lead is known not to form an iodide pbl 4. Lead (iv) chloride is highly unstable towards heat. • lead is known not to form an iodide, p bi 4. Lead(iv) chloride is highly unstable towards heat. Answers (1) on moving. Lead Chloride Is Highly Unstable Towards Heat.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Is Highly Unstable Towards Heat Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. Lead (iv) chloride is highly unstable towards heat. (a) lead belongs to group 14 of the. • lead (iv) chloride is highly unstable towards heat. • lead (ii) chloride reacts with cl 2 to give pbcl 4. • lead (iv) chloride is highly. Lead Chloride Is Highly Unstable Towards Heat.
From www.dreamstime.com
PbCl4 Lead(IV) Chloride CAS 13463304 Chemical Substance in White Lead Chloride Is Highly Unstable Towards Heat • lead is known not to. On moving down group iv, the higher oxidation state becomes unstable because of the inert. Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. • lead (iv) chloride is highly unstable towards heat. (a) lead belongs to group 14 of the. +2 oxidation of pb is. Lead Chloride Is Highly Unstable Towards Heat.
From www.tradeindia.com
Lead Chloride, Lead Chloride Manufacturers & Suppliers, Dealers Lead Chloride Is Highly Unstable Towards Heat • lead is known not to. P b c l 2 + c l 2 → p b c l 4. • lead (ii) chloride reacts with cl 2 to give pbcl 4. • lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. On moving down group iv, the higher oxidation state becomes. Lead Chloride Is Highly Unstable Towards Heat.
From somaap.org
What is an aqueous acid in chemistry, 4 Reactions in Aqueous Solution Lead Chloride Is Highly Unstable Towards Heat • lead is known not to. Lead (iv) chloride is highly unstable towards heat. (b) lead(iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. Lead (ii) chloride reacts with cl 2. Lead Chloride Is Highly Unstable Towards Heat.
From brainly.in
Sodium chloride solution is added to lead nitrate solution. Also write Lead Chloride Is Highly Unstable Towards Heat • lead (iv) chloride is highly unstable towards heat. Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. +2 oxidation of pb is more stable due to inert pair effect. (b) lead(iv) chloride is highly unstable towards heat. Lead is known not to form an iodide pbl 4. Lead (ii) chloride reacts. Lead Chloride Is Highly Unstable Towards Heat.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Is Highly Unstable Towards Heat Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. (a) lead belongs to group 14 of the. • lead (iv) chloride is highly unstable towards heat. On moving down group iv, the higher oxidation state becomes unstable because of the inert. +2 oxidation of pb is more stable due to inert pair. Lead Chloride Is Highly Unstable Towards Heat.
From www.indiamart.com
ACS Powder Lead III Chloride Pure, for Laboratory at Rs 764/gram in Lead Chloride Is Highly Unstable Towards Heat (a) lead belongs to group 14 of the. +2 oxidation of pb is more stable due to inert pair effect. Lead(iv) chloride is highly unstable towards heat. • lead is known not to form an iodide, p bi 4. Lead (iv) chloride is highly unstable towards heat. • lead (iv) chloride is highly unstable towards heat. Lead (ii) chloride reacts. Lead Chloride Is Highly Unstable Towards Heat.
From www.sciencephoto.com
Lead (II) Chloride and Potassium Chromate Stock Image C002/8040 Lead Chloride Is Highly Unstable Towards Heat (b) lead(iv) chloride is highly unstable towards heat. • lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. (a) lead belongs to group 14 of the. Lead (iv) chloride is highly unstable towards heat. Lead is known not to form an iodide pbl 4. +2 oxidation of pb is more stable due to. Lead Chloride Is Highly Unstable Towards Heat.
From www.fishersci.com
Lead(II) chloride, White Crystalline Powder, 99, Alfa Aesar Fisher Lead Chloride Is Highly Unstable Towards Heat • lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. P b c l 2 + c l 2 → p b c l 4. Lead(ii) chloride does not react with `cl_(2)` to give `pbcl_(4)`. • lead is known not to. Lead (iv) chloride is highly unstable towards heat. • lead (ii) chloride. Lead Chloride Is Highly Unstable Towards Heat.
From www.youtube.com
Lead(II) Chloride Synthesis (PbCl2) YouTube Lead Chloride Is Highly Unstable Towards Heat Lead is known not to form an iodide pbl 4. Lead (ii) chloride reacts with cl 2 to give pbcl 4. (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. Lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat.. Lead Chloride Is Highly Unstable Towards Heat.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Is Highly Unstable Towards Heat • lead is known not to form an iodide, p bi 4. Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. Lead is known not to form an iodide pbl 4. Lead(iv) chloride is highly unstable towards heat. • lead (ii) chloride reacts with cl 2 to give pbcl 4. Lead (ii). Lead Chloride Is Highly Unstable Towards Heat.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Is Highly Unstable Towards Heat • lead (ii) chloride reacts with cl 2 to give pbcl 4. • lead is known not to form an iodide, p bi 4. • lead is known not to. (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. On moving down group iv, the higher. Lead Chloride Is Highly Unstable Towards Heat.
From www.toppr.com
Unstable lead compounds are Chemistry Questions Lead Chloride Is Highly Unstable Towards Heat Lead is known not to form an iodide pbl 4. On moving down group iv, the higher oxidation state becomes unstable because of the inert. Lead(ii) chloride does not react with `cl_(2)` to give `pbcl_(4)`. • lead (ii) chloride reacts with cl 2 to give pbcl 4. Lead(iv) chloride is highly unstable towards heat. +2 oxidation of pb is more. Lead Chloride Is Highly Unstable Towards Heat.
From www.gauthmath.com
Solved Drag each statement to the correct location on the chart Lead Chloride Is Highly Unstable Towards Heat Lead (iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. P b c l 2 + c l 2 → p b c l 4. Lead is known not to form an iodide pbl 4. Answers (1) on moving down the group the +4 oxidation of lead has less stable than +2. (b) lead(iv). Lead Chloride Is Highly Unstable Towards Heat.
From www.carolina.com
Lead (II) Chloride, Reagent Grade, 100 g Lead Chloride Is Highly Unstable Towards Heat • lead (iv) chloride is highly unstable towards heat. Lead (ii) chloride reacts with cl 2 to give pbcl 4. (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. (b) lead(iv) chloride is highly unstable towards heat. Lead (iv) chloride is highly unstable towards heat. Lead(iv). Lead Chloride Is Highly Unstable Towards Heat.
From www.gkseries.com
Lead chloride is Lead Chloride Is Highly Unstable Towards Heat (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. • lead is known not to. Lead (iv) chloride is highly unstable towards heat. P b c l 2 + c l 2 → p b c l 4. Lead(iv) chloride is highly unstable towards heat. (a). Lead Chloride Is Highly Unstable Towards Heat.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Is Highly Unstable Towards Heat Lead (iv) chloride is highly unstable towards heat. P b c l 2 + c l 2 → p b c l 4. (ii) lead tetrachloride ( p b c l 4) is highly unstable towards heat because the stability of +4 oxidation state decreases on. • lead is known not to. Lead (iv) chloride is highly unstable towards heat.. Lead Chloride Is Highly Unstable Towards Heat.