Does The Coefficient Change The Identity Of A Compound . The coefficient tells you how many molecules of that substance there is. The subscript tells you what the substance it. Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). In some chemical formulas it is necessary to use parentheses. You cannot change subscripts in a chemical formula to balance a chemical equation; You can change only the coefficients. The subscript outside the parentheses refers to all the elements inside. Coefficients must be whole numbers and. Changing a coefficient changes the amount of substance but not its identity or properties. In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements.
from www.slideserve.com
To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. Changing a coefficient changes the amount of substance but not its identity or properties. You can change only the coefficients. You cannot change subscripts in a chemical formula to balance a chemical equation; The subscript tells you what the substance it. In some chemical formulas it is necessary to use parentheses. The subscript outside the parentheses refers to all the elements inside. Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. Coefficients must be whole numbers and.
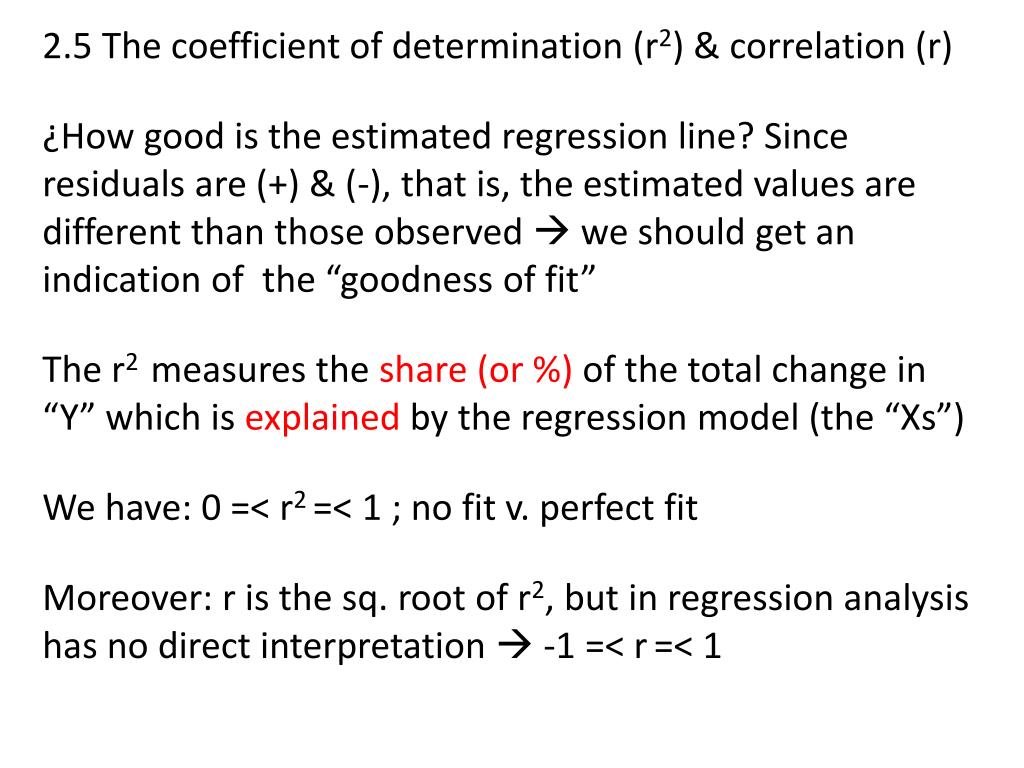
PPT 2.5 The coefficient of determination (r 2 ) & correlation (r
Does The Coefficient Change The Identity Of A Compound You cannot change subscripts in a chemical formula to balance a chemical equation; Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. In some chemical formulas it is necessary to use parentheses. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). Changing a coefficient changes the amount of substance but not its identity or properties. The coefficient tells you how many molecules of that substance there is. You can change only the coefficients. The subscript outside the parentheses refers to all the elements inside. You cannot change subscripts in a chemical formula to balance a chemical equation; The subscript tells you what the substance it. In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. Coefficients must be whole numbers and.
From www.scribd.com
distribution coefficient Solution Phase (Matter) Does The Coefficient Change The Identity Of A Compound The subscript outside the parentheses refers to all the elements inside. Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. Changing a coefficient changes the amount of substance but not its identity or properties. The subscript tells you what the substance it. In a chemical equation representing a chemical reaction what do. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Regression Models PowerPoint Presentation, free download ID292477 Does The Coefficient Change The Identity Of A Compound In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. The subscript outside the parentheses refers to all the elements inside. Changing a coefficient changes the amount of substance but not its identity or properties. You cannot change subscripts in a chemical formula to balance a chemical equation; To. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT The Standard Regression Model and its Spatial Alternatives Does The Coefficient Change The Identity Of A Compound To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). In some chemical formulas it is necessary to use parentheses. Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. You can change only the coefficients. Changing a coefficient changes the amount of substance but. Does The Coefficient Change The Identity Of A Compound.
From www.cuemath.com
Definition of coefficient with examples Cuemath Does The Coefficient Change The Identity Of A Compound In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. You cannot change subscripts in a chemical formula to balance a chemical equation; Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. The coefficient tells you how many molecules of that. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Process Control Quality Control for Quantitative Tests Does The Coefficient Change The Identity Of A Compound The subscript tells you what the substance it. The subscript outside the parentheses refers to all the elements inside. Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. Changing a coefficient. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT SIMPLE REGRESSION AND CORRELATION PowerPoint Presentation, free Does The Coefficient Change The Identity Of A Compound You can change only the coefficients. In some chemical formulas it is necessary to use parentheses. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). Coefficients must be whole numbers and. The subscript outside the parentheses refers to all the elements inside. Both coefficient and subscript refer to numbers, but they. Does The Coefficient Change The Identity Of A Compound.
From www.sliderbase.com
Steps to Balancing Equations Does The Coefficient Change The Identity Of A Compound The subscript outside the parentheses refers to all the elements inside. You cannot change subscripts in a chemical formula to balance a chemical equation; Changing a coefficient changes the amount of substance but not its identity or properties. Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. In a chemical equation representing. Does The Coefficient Change The Identity Of A Compound.
From ar.inspiredpencil.com
What Is A Coefficient In Math Does The Coefficient Change The Identity Of A Compound To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). Coefficients must be whole numbers and. The subscript tells you what the substance it. You cannot change subscripts in a chemical formula to balance a chemical equation; Changing a coefficient changes the amount of substance but not its identity or properties. In. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT The Coefficient of Determination PowerPoint Presentation, free Does The Coefficient Change The Identity Of A Compound The coefficient tells you how many molecules of that substance there is. Coefficients must be whole numbers and. You cannot change subscripts in a chemical formula to balance a chemical equation; The subscript outside the parentheses refers to all the elements inside. In some chemical formulas it is necessary to use parentheses. The subscript tells you what the substance it.. Does The Coefficient Change The Identity Of A Compound.
From www.youtube.com
Reflection Coefficient — Lesson 7 YouTube Does The Coefficient Change The Identity Of A Compound Coefficients must be whole numbers and. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). The coefficient tells you how many molecules of that substance there is. Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. The subscript tells you what the substance. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT The Design of Dosage Form PowerPoint Presentation, free download Does The Coefficient Change The Identity Of A Compound You cannot change subscripts in a chemical formula to balance a chemical equation; Changing a coefficient changes the amount of substance but not its identity or properties. Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. To do so would change the chemical identity of the species being described, as illustrated in. Does The Coefficient Change The Identity Of A Compound.
From www.youtube.com
How to change coefficient to balance chemical equations. YouTube Does The Coefficient Change The Identity Of A Compound In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. The subscript tells you what the substance it. In some chemical formulas it is necessary to use parentheses. The subscript outside the parentheses refers to all the elements inside. You can change only the coefficients. Changing a coefficient changes. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT JouleThomson Coefficient PowerPoint Presentation, free download Does The Coefficient Change The Identity Of A Compound You cannot change subscripts in a chemical formula to balance a chemical equation; In some chemical formulas it is necessary to use parentheses. In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. The coefficient tells you how many molecules of that substance there is. The subscript outside the. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Dimensional Analysis and Similitude PowerPoint Presentation, free Does The Coefficient Change The Identity Of A Compound The subscript outside the parentheses refers to all the elements inside. In some chemical formulas it is necessary to use parentheses. You can change only the coefficients. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). Changing a coefficient changes the amount of substance but not its identity or properties. In. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Pharmaceutical Medicinal Chemistry1 PowerPoint Presentation Does The Coefficient Change The Identity Of A Compound Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. The coefficient tells you how many molecules of that substance there is. You cannot change subscripts in a chemical formula to balance a chemical equation; To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\).. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT How to Balance Chemical Equations PowerPoint Presentation, free Does The Coefficient Change The Identity Of A Compound To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). Changing a coefficient changes the amount of substance but not its identity or properties. You cannot change subscripts in a chemical formula to balance a chemical equation; Both coefficient and subscript refer to numbers, but they give different details about a particular. Does The Coefficient Change The Identity Of A Compound.
From www.youtube.com
Trigonometry Compound Angle Formula and Derivations YouTube Does The Coefficient Change The Identity Of A Compound Coefficients must be whole numbers and. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. You can change only the coefficients. The coefficient tells you how many molecules of that. Does The Coefficient Change The Identity Of A Compound.
From www.tessshebaylo.com
Coefficient And Subscript In A Chemical Equation Tessshebaylo Does The Coefficient Change The Identity Of A Compound In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. Changing a coefficient changes the amount of substance but not its identity or properties. In some chemical formulas it is necessary to use parentheses. The subscript tells you what the substance it. Both coefficient and subscript refer to numbers,. Does The Coefficient Change The Identity Of A Compound.
From www.pdf-archive.com
Day 10 Binomial coefficient identities PDF Archive Does The Coefficient Change The Identity Of A Compound In some chemical formulas it is necessary to use parentheses. The coefficient tells you how many molecules of that substance there is. In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. The subscript tells you what the substance it. The subscript outside the parentheses refers to all the. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Introduction to Chemical Equations PowerPoint Presentation, free Does The Coefficient Change The Identity Of A Compound The subscript outside the parentheses refers to all the elements inside. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). The coefficient tells you how many molecules of that substance there is. In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Chapter 6 Chemical Reactions PowerPoint Presentation, free Does The Coefficient Change The Identity Of A Compound Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). The subscript tells you what the substance it. The coefficient tells you how many molecules of that substance there is. Coefficients must be whole numbers. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Does The Coefficient Change The Identity Of A Compound To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). You cannot change subscripts in a chemical formula to balance a chemical equation; In some chemical formulas it is necessary to use parentheses. The subscript tells you what the substance it. Both coefficient and subscript refer to numbers, but they give different. Does The Coefficient Change The Identity Of A Compound.
From www.cambridge.org
87.34 Some binomial coefficient identities The Mathematical Gazette Does The Coefficient Change The Identity Of A Compound Coefficients must be whole numbers and. The coefficient tells you how many molecules of that substance there is. In some chemical formulas it is necessary to use parentheses. The subscript outside the parentheses refers to all the elements inside. In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements.. Does The Coefficient Change The Identity Of A Compound.
From www.studypug.com
Master Balancing Chemical Equations StepbyStep Guide StudyPug Does The Coefficient Change The Identity Of A Compound Changing a coefficient changes the amount of substance but not its identity or properties. The subscript tells you what the substance it. The coefficient tells you how many molecules of that substance there is. Coefficients must be whole numbers and. You cannot change subscripts in a chemical formula to balance a chemical equation; The subscript outside the parentheses refers to. Does The Coefficient Change The Identity Of A Compound.
From sciencenotes.org
What Is a Chemical Equation? Definition and Examples Does The Coefficient Change The Identity Of A Compound The subscript outside the parentheses refers to all the elements inside. Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. Coefficients must be whole numbers and. In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. You cannot change subscripts in. Does The Coefficient Change The Identity Of A Compound.
From programmathically.com
Identity Matrix and Inverse Matrix Programmathically Does The Coefficient Change The Identity Of A Compound In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. Coefficients must be whole numbers and. Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. In some chemical formulas it is necessary to use parentheses. The subscript outside the parentheses refers. Does The Coefficient Change The Identity Of A Compound.
From towardsdatascience.com
What are Covariance and Correlation coefficients and their significance Does The Coefficient Change The Identity Of A Compound The subscript tells you what the substance it. You cannot change subscripts in a chemical formula to balance a chemical equation; To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). The subscript outside the parentheses refers to all the elements inside. Both coefficient and subscript refer to numbers, but they give. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Partition Coefficients PowerPoint Presentation, free download Does The Coefficient Change The Identity Of A Compound Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. The subscript outside the parentheses refers to all the elements inside. The coefficient tells you how many molecules of that substance there is. You cannot change subscripts in a chemical formula to balance a chemical equation; The subscript tells you what the substance. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT 2.5 The coefficient of determination (r 2 ) & correlation (r Does The Coefficient Change The Identity Of A Compound You can change only the coefficients. In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of compound and elements. Changing a coefficient changes the amount of substance but not its identity or properties. The coefficient tells you how many molecules of that substance there is. The subscript outside the parentheses refers to. Does The Coefficient Change The Identity Of A Compound.
From brainly.com
Reactants undergo chemical reaction to form products. This chemical Does The Coefficient Change The Identity Of A Compound Coefficients must be whole numbers and. The coefficient tells you how many molecules of that substance there is. You can change only the coefficients. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). In a chemical equation representing a chemical reaction what do the numbers in front of the molecules of. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Coefficient of Variation PowerPoint Presentation, free download Does The Coefficient Change The Identity Of A Compound Both coefficient and subscript refer to numbers, but they give different details about a particular chemical reaction. The coefficient tells you how many molecules of that substance there is. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). In a chemical equation representing a chemical reaction what do the numbers in. Does The Coefficient Change The Identity Of A Compound.
From www.numerade.com
SOLVED The partition coefficient of Compound X is 11. Using the Does The Coefficient Change The Identity Of A Compound Coefficients must be whole numbers and. You can change only the coefficients. You cannot change subscripts in a chemical formula to balance a chemical equation; The coefficient tells you how many molecules of that substance there is. The subscript tells you what the substance it. The subscript outside the parentheses refers to all the elements inside. To do so would. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Reactivity Coefficients PowerPoint Presentation, free download Does The Coefficient Change The Identity Of A Compound Changing a coefficient changes the amount of substance but not its identity or properties. The coefficient tells you how many molecules of that substance there is. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). You cannot change subscripts in a chemical formula to balance a chemical equation; The subscript tells. Does The Coefficient Change The Identity Of A Compound.
From www.slideserve.com
PPT Understanding Equity Statistics PowerPoint Presentation, free Does The Coefficient Change The Identity Of A Compound You can change only the coefficients. To do so would change the chemical identity of the species being described, as illustrated in figure \(\pageindex{3}\). You cannot change subscripts in a chemical formula to balance a chemical equation; In some chemical formulas it is necessary to use parentheses. The coefficient tells you how many molecules of that substance there is. The. Does The Coefficient Change The Identity Of A Compound.
From www.youtube.com
What is a Coefficient? YouTube Does The Coefficient Change The Identity Of A Compound Coefficients must be whole numbers and. The subscript outside the parentheses refers to all the elements inside. Changing a coefficient changes the amount of substance but not its identity or properties. You can change only the coefficients. You cannot change subscripts in a chemical formula to balance a chemical equation; The coefficient tells you how many molecules of that substance. Does The Coefficient Change The Identity Of A Compound.