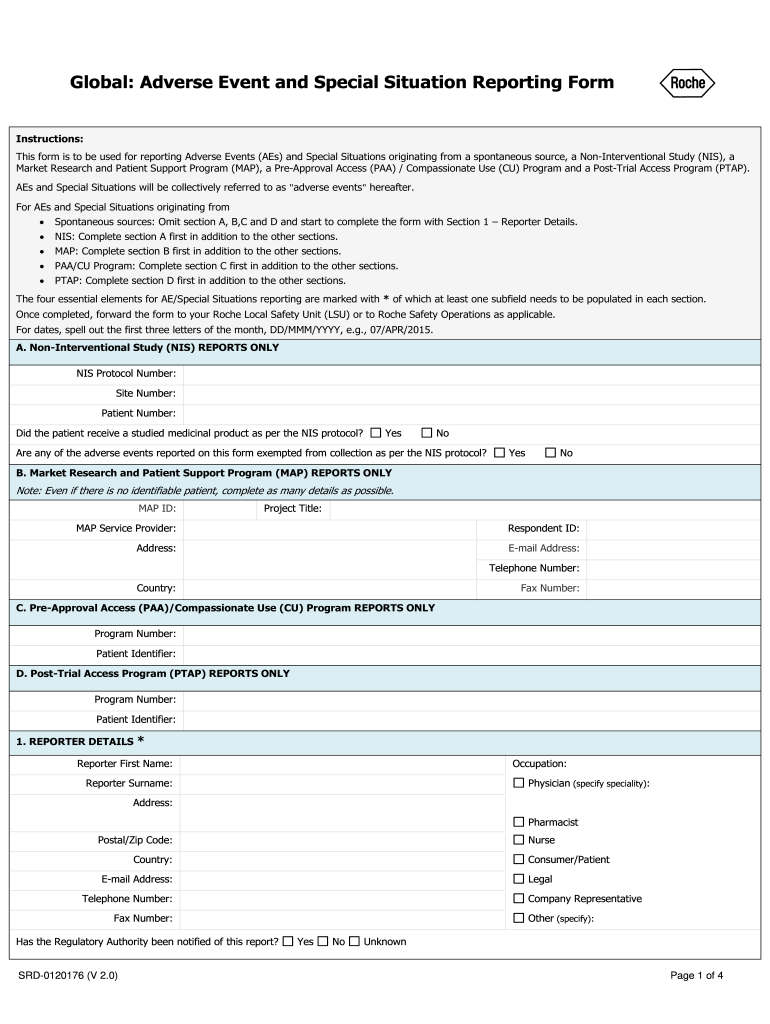
Mhra Medical Devices inar . The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. Learn about uk mhra's registration process for medical devices and ivds. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response.
from exowgjrmc.blob.core.windows.net
Learn about uk mhra's registration process for medical devices and ivds. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response.
Mhra Medical Devices Incident Reporting at Leticia Ridley blog
Mhra Medical Devices inar Learn about uk mhra's registration process for medical devices and ivds. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. Learn about uk mhra's registration process for medical devices and ivds. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential.
From exowgjrmc.blob.core.windows.net
Mhra Medical Devices Incident Reporting at Leticia Ridley blog Mhra Medical Devices inar On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response.. Mhra Medical Devices inar.
From www.podymos.com
12 Tips for Running Medical Device inars in 2022 Podymos Mhra Medical Devices inar Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. Learn about uk mhra's registration process for medical devices and ivds. On 9. Mhra Medical Devices inar.
From exowgjrmc.blob.core.windows.net
Mhra Medical Devices Incident Reporting at Leticia Ridley blog Mhra Medical Devices inar Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. Learn about uk mhra's registration process for medical devices and ivds. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds,. Mhra Medical Devices inar.
From operonstrategist.com
A Comprehensive Guide to MHRA Medical Device Registration (Steps Mhra Medical Devices inar The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published. Mhra Medical Devices inar.
From www.greenlight.guru
How MHRA is Regulating Medical Devices in the UK after Brexit Mhra Medical Devices inar On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Medical. Mhra Medical Devices inar.
From fyospdvnx.blob.core.windows.net
Mhra Medical Devices Login at Beatrice Geraghty blog Mhra Medical Devices inar On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and.. Mhra Medical Devices inar.
From www.cognidox.com
New IVD regulation is coming. are you ready? Mhra Medical Devices inar Learn about uk mhra's registration process for medical devices and ivds. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. We also. Mhra Medical Devices inar.
From mdlaw.eu
inar Step by Step MHRA Registration & Clinical Testing · MDlaw Mhra Medical Devices inar Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential.. Mhra Medical Devices inar.
From exoitvqua.blob.core.windows.net
Mhra Medical Devices Portal at Patricia Caudle blog Mhra Medical Devices inar On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. Learn about uk mhra's registration process for medical devices. Mhra Medical Devices inar.
From www.gov.uk
MHRA increases UK assessment capacity for invitro diagnostic devices Mhra Medical Devices inar The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Learn about uk mhra's registration process for medical devices and ivds. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. On sunday 26 june the medicines and healthcare products. Mhra Medical Devices inar.
From www.interactivesoftware.co.uk
inar How a LIMS Supports HTA and MHRA Compliance Mhra Medical Devices inar The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. Learn about uk mhra's registration process for medical devices and ivds. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. We also. Mhra Medical Devices inar.
From helpline.meditrial.net
UK MHRA provides regulatory guidance on software used in the diagnosis Mhra Medical Devices inar Learn about uk mhra's registration process for medical devices and ivds. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk. Mhra Medical Devices inar.
From www.gandlhealth.com
MHRA announces changes to medical device regulations G&L Healthcare Mhra Medical Devices inar On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. The medicines and healthcare products regulatory agency (mhra) is. Mhra Medical Devices inar.
From operonstrategist.com
A Comprehensive Guide to MHRA Medical Device Registration (Steps Mhra Medical Devices inar Learn about uk mhra's registration process for medical devices and ivds. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap. Mhra Medical Devices inar.
From www.youtube.com
StepbyStep Guide How to Get UK MHRA Registration for Medical Devices Mhra Medical Devices inar The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. Learn about uk mhra's registration process for medical devices and. Mhra Medical Devices inar.
From www.linkedin.com
MHRA publish an update on future medical device regulations Mhra Medical Devices inar On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. The medicines and healthcare products regulatory agency (mhra) is. Mhra Medical Devices inar.
From helpline.meditrial.net
UK MHRA updates guidance on virtual manufacturing of medical devices Mhra Medical Devices inar On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. Learn about uk mhra's registration process for medical devices and ivds. Medical devices regulation webinar this webinar will inform. Mhra Medical Devices inar.
From www.regdesk.co
MHRA Consultation on the Future Regulation of Medical Devices in the UK Mhra Medical Devices inar The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. Learn about uk mhra's registration process for medical devices and ivds. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. On 9. Mhra Medical Devices inar.
From heliovigil.com
Notify the MHRA about a clinical investigation for a medical device Mhra Medical Devices inar Learn about uk mhra's registration process for medical devices and ivds. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its. Mhra Medical Devices inar.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Mhra Medical Devices inar On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. Learn about uk mhra's registration process for medical devices and ivds. On sunday 26 june the medicines and healthcare. Mhra Medical Devices inar.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Mhra Medical Devices inar On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. On sunday 26 june. Mhra Medical Devices inar.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Mhra Medical Devices inar We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. Learn about uk mhra's registration process for medical devices and ivds. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its. Mhra Medical Devices inar.
From www.emergobyul.com
MHRA, U.K. Road Map and Swiss Regulatory Updates Emergo by UL Mhra Medical Devices inar On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. We also held a. Mhra Medical Devices inar.
From mediapigeon.io
MHRA appoints first new UK Approved Body to certify medical devices Mhra Medical Devices inar On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Medical devices regulation webinar. Mhra Medical Devices inar.
From medium.com
MHRA Medical Device Registration Everything You Need to Know by Mhra Medical Devices inar The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. Learn about uk mhra's registration process for medical devices and ivds. On 9. Mhra Medical Devices inar.
From www.scribd.com
Managing Medical Devices MHRA Medical Device Reliability Engineering Mhra Medical Devices inar We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical.. Mhra Medical Devices inar.
From exoitvqua.blob.core.windows.net
Mhra Medical Devices Portal at Patricia Caudle blog Mhra Medical Devices inar Learn about uk mhra's registration process for medical devices and ivds. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk. Mhra Medical Devices inar.
From woodleybioreg.com
MHRA outlines roadmap for new medical device regulations Woodley BioReg Mhra Medical Devices inar Learn about uk mhra's registration process for medical devices and ivds. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds,. Mhra Medical Devices inar.
From www.bhta.com
MHRA Consultation on the Future Regulation of Medical Devices in the UK Mhra Medical Devices inar The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. Learn about uk mhra's registration process for medical devices and ivds. On 9. Mhra Medical Devices inar.
From casusconsulting.com
2024 UK MHRA Registration StepbyStep Guide Casus Consulting Mhra Medical Devices inar The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. We also held a webinar which covered the intentions regarding scope, classification of. Mhra Medical Devices inar.
From exowgjrmc.blob.core.windows.net
Mhra Medical Devices Incident Reporting at Leticia Ridley blog Mhra Medical Devices inar On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Learn about uk mhra's registration process for medical devices and. Mhra Medical Devices inar.
From www.my-conect.com
Common Mistakes to Avoid When Registering Your Medical Devices with MHRA Mhra Medical Devices inar Learn about uk mhra's registration process for medical devices and ivds. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. On 9. Mhra Medical Devices inar.
From www.scribd.com
MHRA Medical Devices PDF PDF Clinical Trial Medical Device Mhra Medical Devices inar We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and.. Mhra Medical Devices inar.
From exofautol.blob.core.windows.net
Mhra Medical Devices Research at Tracy Messier blog Mhra Medical Devices inar Learn about uk mhra's registration process for medical devices and ivds. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the government response. On 9 january 2024, the medicines and healthcare products regulatory agency (mhra) published its roadmap for the delivery of the future regulatory framework for medical. The medicines and healthcare products regulatory agency (mhra). Mhra Medical Devices inar.
From www.lexology.com
The MHRA's recent updates to the regulation of medical devices Lexology Mhra Medical Devices inar Learn about uk mhra's registration process for medical devices and ivds. Medical devices regulation webinar this webinar will inform stakeholder groups on timelines of regulation development and. We also held a webinar which covered the intentions regarding scope, classification of general medical devices and ivds, essential. On sunday 26 june the medicines and healthcare products regulatory agency (mhra) published the. Mhra Medical Devices inar.