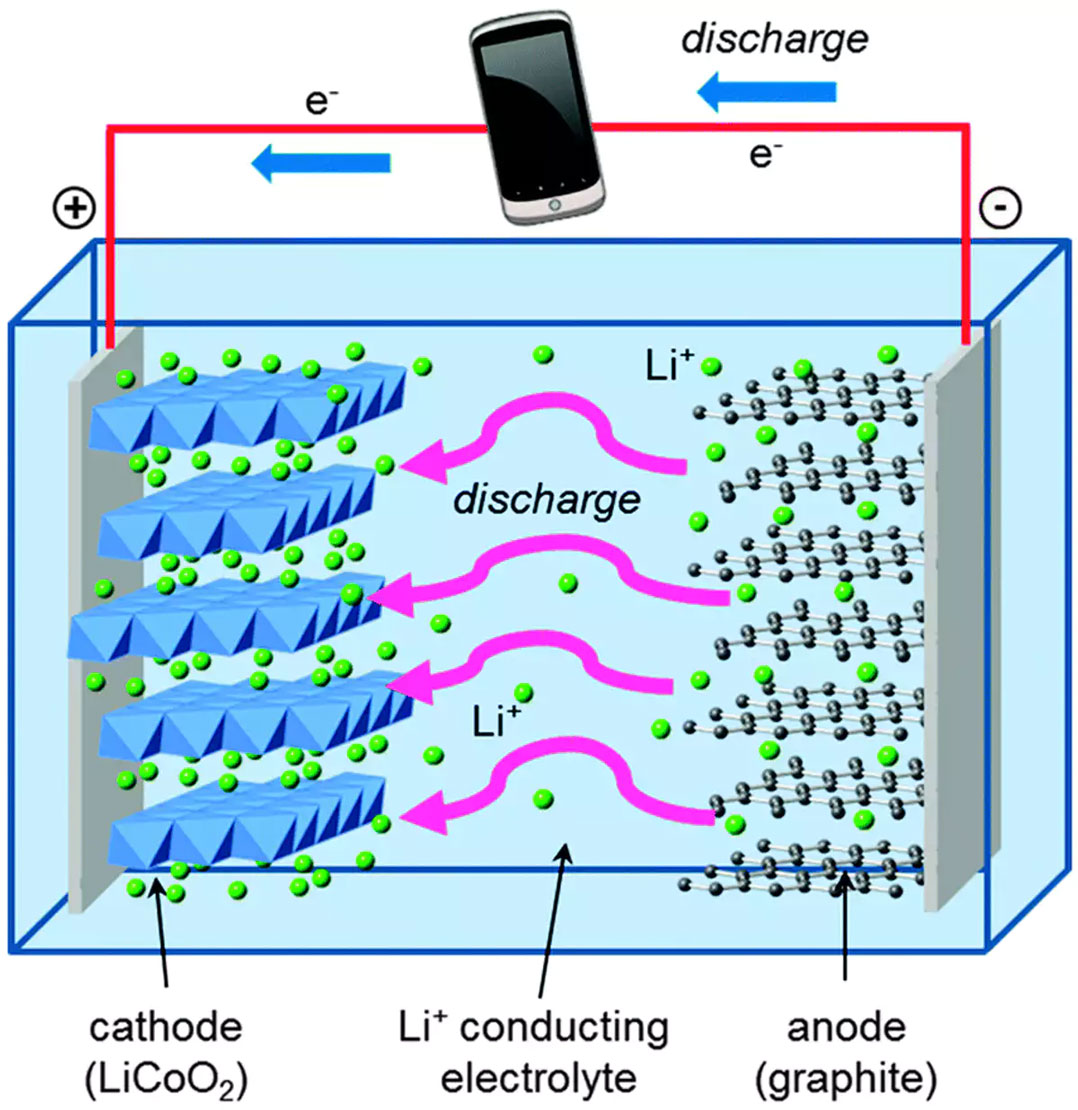
Electrons In A Battery . There are three main components of a battery: Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. The flow of electrons provides an. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Batteries work by making these electrons move from one part of. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. Two terminals made of different chemicals (typically metals), the anode and the. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the.
from techcentral.co.za
When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. There are three main components of a battery: Two terminals made of different chemicals (typically metals), the anode and the. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. Batteries work by making these electrons move from one part of. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. The flow of electrons provides an.
Explainer How lithiumion batteries work TechCentral
Electrons In A Battery The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. Batteries work by making these electrons move from one part of. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. There are three main components of a battery: The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. The flow of electrons provides an. Two terminals made of different chemicals (typically metals), the anode and the.
From mybios.me
How Electrons Flow In A Battery Circuit Bios Pics Electrons In A Battery Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. Two terminals made of different chemicals (typically metals), the anode and the. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. The electrodes must be different materials with. Electrons In A Battery.
From www.linkedin.com
Understanding the Flow of Electrons in Charging LithiumIon Batteries Electrons In A Battery The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. The flow of electrons provides an. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. Scientists. Electrons In A Battery.
From www.batterypowertips.com
What is a cathode? Battery Power Tips Electrons In A Battery The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. The flow of electrons provides an. There are three main components of a battery: Two terminals made of different chemicals (typically metals), the anode and the. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another,. Electrons In A Battery.
From www.dreamstime.com
Dry Cell. Cross Section of Battery with Cathode, Anode, Paste, Light Electrons In A Battery Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. The flow of electrons provides an. Two terminals made of different chemicals (typically metals), the anode and the. Batteries work by making these electrons move from one part of. When you connect a battery's. Electrons In A Battery.
From www.dailymail.co.uk
Magnesium could be the key to batteries that don't explode Daily Mail Electrons In A Battery There are three main components of a battery: The flow of electrons provides an. Batteries work by making these electrons move from one part of. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. For example, they are developing improved materials for the. Electrons In A Battery.
From www.slideserve.com
PPT Electrons flow from the __ terminal of the battery, through the Electrons In A Battery Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. The flow of electrons provides an. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. When you connect a battery's two electrodes into a circuit (for example, when. Electrons In A Battery.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Electrons In A Battery Two terminals made of different chemicals (typically metals), the anode and the. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Batteries work by making these electrons move from one part of. The flow of electrons provides an. There are three main components of a battery: Scientists. Electrons In A Battery.
From www.alamy.com
Liion battery diagram. Vector illustration. Rechargeable battery in Electrons In A Battery When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. The chemical reactions in a battery. Electrons In A Battery.
From dannymeta.com
What creates an electric current in a battery Electrons In A Battery When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the.. Electrons In A Battery.
From www.linuxfriends.net
Solar Energy Introduction Course Electrons In A Battery Batteries work by making these electrons move from one part of. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. There are three main components of a battery: Two terminals made of different chemicals (typically metals), the anode and the. The chemical reactions in a battery involve. Electrons In A Battery.
From www.brookings.edu
Five emerging battery technologies for electric vehicles Electrons In A Battery There are three main components of a battery: The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. Scientists are using new tools to better understand the electrical and chemical processes in. Electrons In A Battery.
From www.youtube.com
The Electric Battery and Conventional Current Introduction to Basic Electrons In A Battery The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. The flow of electrons provides an. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries.. Electrons In A Battery.
From quizlet.com
**Describe** how a battery causes electrons to move in a cir Quizlet Electrons In A Battery Two terminals made of different chemicals (typically metals), the anode and the. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. Batteries work by making these electrons move. Electrons In A Battery.
From quizlet.com
Your friend says that a battery supplies the electrons in an Quizlet Electrons In A Battery Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. Batteries work by making these electrons move from one part of. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. The chemical reactions in a battery. Electrons In A Battery.
From www.electricity-magnetism.org
Lithiumion Battery How it works Reaction, Anode & Cathode Electrons In A Battery The flow of electrons provides an. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. There are three main components of a battery: Batteries work by making these electrons move from one part. Electrons In A Battery.
From www.jeh-tech.com
Batteries notes Electrons In A Battery When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. Two terminals made of different chemicals (typically metals), the anode and the. Batteries work by making these electrons move from one. Electrons In A Battery.
From www.pinterest.com
The chemical reactions in the battery causes a build up of electrons at Electrons In A Battery Two terminals made of different chemicals (typically metals), the anode and the. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. There are three main components of a. Electrons In A Battery.
From techcentral.co.za
Explainer How lithiumion batteries work TechCentral Electrons In A Battery The flow of electrons provides an. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the.. Electrons In A Battery.
From www.researchgate.net
Schematic illustration of a lithiumion battery (LIB) under discharge Electrons In A Battery For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. The flow of electrons provides an. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Two terminals made of different chemicals (typically metals), the anode and the. The electrodes must be different. Electrons In A Battery.
From www.pinterest.com
Figure 1 In a battery, the flow of electrons goes from anode to Electrons In A Battery Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. Two terminals made of different chemicals (typically metals), the anode and the. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. The flow of electrons provides an. There. Electrons In A Battery.
From www.comsol.com
Does the Current Flow Backwards Inside a Battery? COMSOL Blog Electrons In A Battery When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Batteries work by making these electrons move from one part of. There are three main components of a battery: The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an. Electrons In A Battery.
From fphoto.photoshelter.com
science electricity simple circuit battery Fundamental Photographs Electrons In A Battery The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Two terminals made of different chemicals (typically metals), the anode and the. Scientists are using new tools to better understand the. Electrons In A Battery.
From instrumentationtools.com
Batteries Theory Inst Tools Electrons In A Battery For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. The flow of electrons provides an. There are three main components of a battery: The electrodes must be different. Electrons In A Battery.
From www.slideserve.com
PPT Electricity PowerPoint Presentation, free download ID7008919 Electrons In A Battery When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. There are three main components of a battery: Batteries work by making these electrons move from one part of. The flow of electrons provides an. Two terminals made of different chemicals (typically metals), the anode and the. For. Electrons In A Battery.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrons In A Battery Two terminals made of different chemicals (typically metals), the anode and the. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to. Electrons In A Battery.
From blog.upsbatterycenter.com
The Flow of Electrons Inside Battery Cells News about Energy Storage Electrons In A Battery There are three main components of a battery: The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. Batteries work by making these electrons move from one part of. Scientists are using new tools. Electrons In A Battery.
From technologystudent.com
Batteries and LEDs Electrons In A Battery Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. Batteries work by making these electrons move from one part of. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. For example, they are developing improved. Electrons In A Battery.
From energyknowledgebase.com
Electricity · Energy KnowledgeBase Electrons In A Battery The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. Two terminals made of different chemicals (typically metals), the anode and the. The flow of electrons provides an. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. When you connect a. Electrons In A Battery.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode Electrons In A Battery For example, they are developing improved materials for the anodes, cathodes, and electrolytes in batteries. Batteries work by making these electrons move from one part of. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. The flow of electrons provides an. There are three main components of. Electrons In A Battery.
From www.istockphoto.com
110+ Electron Battery Physics Electricity Stock Photos, Pictures Electrons In A Battery Two terminals made of different chemicals (typically metals), the anode and the. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. The flow of electrons provides an. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation. Electrons In A Battery.
From www.pinterest.com
How A Battery Works Chemical reactions, It works, Learning Electrons In A Battery The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. Two terminals made of different chemicals (typically metals), the anode and the. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. There. Electrons In A Battery.
From skill-lync.com
Week 1 Understanding Different Battery Chemistry SkillLync Electrons In A Battery The flow of electrons provides an. The electrodes must be different materials with different chemical reactivity to allow electrons to move round the. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Scientists are using new tools to better understand the electrical and chemical processes in batteries. Electrons In A Battery.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Electrons In A Battery Two terminals made of different chemicals (typically metals), the anode and the. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. Batteries work by making these electrons move from one part of. When you connect a battery's two electrodes into a circuit (for example, when you put one. Electrons In A Battery.
From thepowerfacts.com
How Do Batteries Create Electricity? Here is the Reaction! The Power Electrons In A Battery Batteries work by making these electrons move from one part of. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy storage. For. Electrons In A Battery.
From www.dreamstime.com
Electrons Flow on Cooper Wire, Learning Electricity Stock Illustration Electrons In A Battery When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Batteries work by making these electrons move from one part of. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. For example, they are developing improved. Electrons In A Battery.