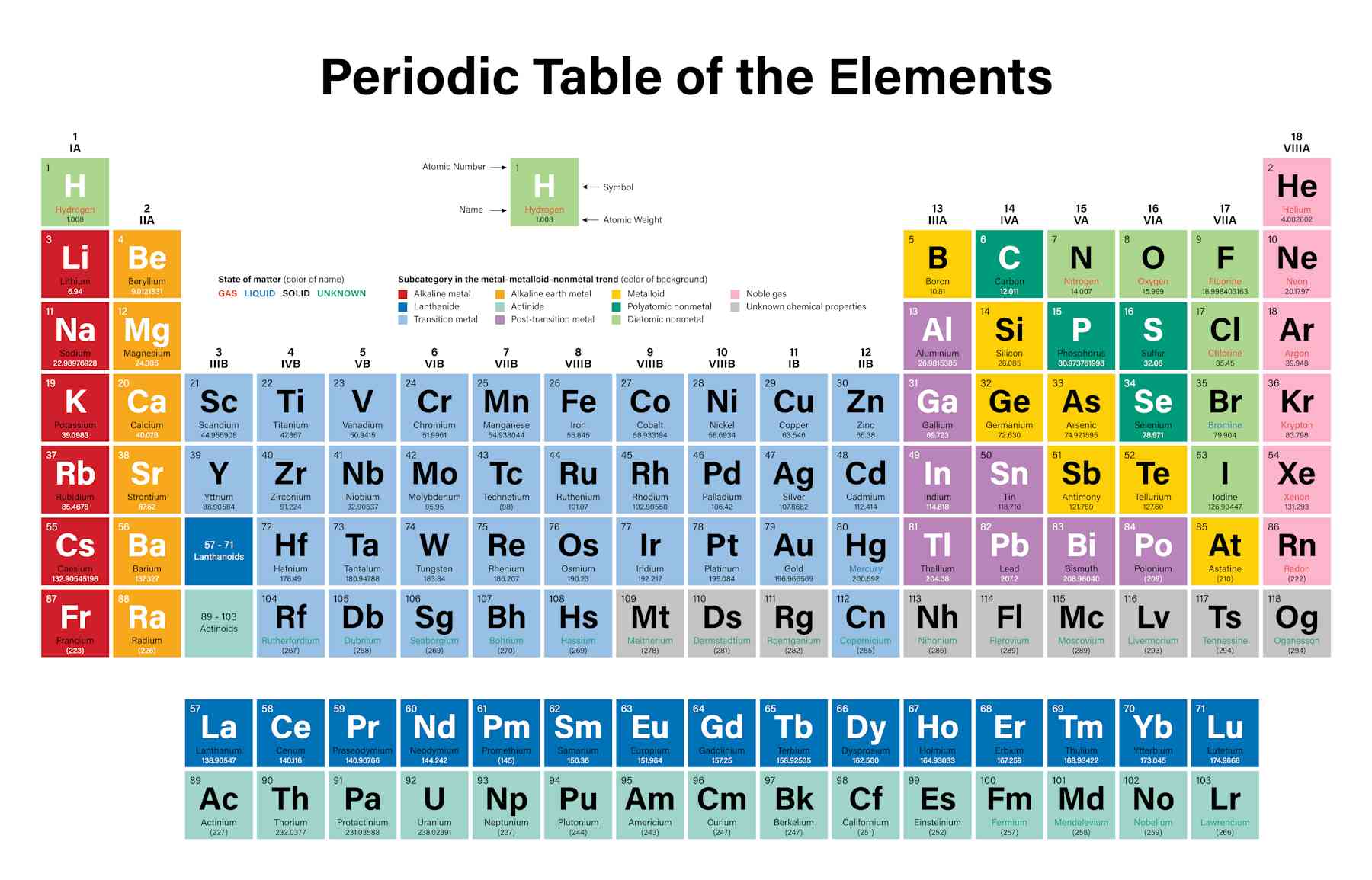
Are Metal Dull . Properties of metalloids or semimetals. Elements may be classified as metals, nonmetals, or metalloids. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Elements with some characteristics of metals. Metalloids have some of the properties of metals and some nonmetallic characteristics. metals vs nonmetals. iridium and osmium have the highest densities whereas lithium has the lowest density. Thus, they are electropositive elements. Metals have high melting and boiling. By anne marie helmenstine, ph.d. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. not lustrous (dull appearance) poor conductors of heat and electricity. Examples of metalloids include boron, silicon, and arsenic.
from theconversation.com
Examples of metalloids include boron, silicon, and arsenic. Thus, they are electropositive elements. Properties of metalloids or semimetals. By anne marie helmenstine, ph.d. Elements with some characteristics of metals. not lustrous (dull appearance) poor conductors of heat and electricity. Metalloids have some of the properties of metals and some nonmetallic characteristics. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. iridium and osmium have the highest densities whereas lithium has the lowest density. metals vs nonmetals.
Understanding the periodic table through the lens of the volatile Group
Are Metal Dull Examples of metalloids include boron, silicon, and arsenic. Properties of metalloids or semimetals. iridium and osmium have the highest densities whereas lithium has the lowest density. Elements with some characteristics of metals. Elements may be classified as metals, nonmetals, or metalloids. Examples of metalloids include boron, silicon, and arsenic. Thus, they are electropositive elements. metals vs nonmetals. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. not lustrous (dull appearance) poor conductors of heat and electricity. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. By anne marie helmenstine, ph.d. Metals have high melting and boiling. Metalloids have some of the properties of metals and some nonmetallic characteristics.
From jaceybildean.blogspot.com
Is Sulfur a Metal JaceybilDean Are Metal Dull Metalloids have some of the properties of metals and some nonmetallic characteristics. Elements may be classified as metals, nonmetals, or metalloids. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. Examples of metalloids include boron, silicon, and arsenic. metals vs nonmetals. All elements except hydrogen, which form positive ions by losing electrons. Are Metal Dull.
From freepbr.com
Metals Archives Free PBR Materials Are Metal Dull Properties of metalloids or semimetals. Metals have high melting and boiling. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. not lustrous (dull appearance) poor conductors of heat and electricity. Elements with some characteristics of metals. Elements may be classified as metals, nonmetals, or metalloids. Metalloids have some of the properties. Are Metal Dull.
From opengeology.org
Earth Materials Mineral Identification Historical Geology Are Metal Dull not lustrous (dull appearance) poor conductors of heat and electricity. Properties of metalloids or semimetals. Elements may be classified as metals, nonmetals, or metalloids. Metalloids have some of the properties of metals and some nonmetallic characteristics. Metals have high melting and boiling. iridium and osmium have the highest densities whereas lithium has the lowest density. metals vs. Are Metal Dull.
From www.diamondvogel.com
Dull Metallic Are Metal Dull Properties of metalloids or semimetals. Elements with some characteristics of metals. iridium and osmium have the highest densities whereas lithium has the lowest density. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. Examples of metalloids include boron, silicon, and arsenic. Thus, they are electropositive elements. Elements may be classified as metals,. Are Metal Dull.
From www.hardwareandtools.com
Krylon 1403 Metallic Spray Paint Dull Aluminum Metallic 11 Ounce Are Metal Dull Thus, they are electropositive elements. By anne marie helmenstine, ph.d. iridium and osmium have the highest densities whereas lithium has the lowest density. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. not lustrous (dull appearance) poor conductors of heat and electricity. Elements may be classified as metals, nonmetals, or. Are Metal Dull.
From www.alamy.com
Dull metal hires stock photography and images Alamy Are Metal Dull iridium and osmium have the highest densities whereas lithium has the lowest density. Thus, they are electropositive elements. metals vs nonmetals. Properties of metalloids or semimetals. Elements with some characteristics of metals. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. not lustrous (dull appearance) poor conductors of heat. Are Metal Dull.
From www.artstation.com
ArtStation Metal Steel Dull Seamless PBR Texture by Erica Grenci Are Metal Dull By anne marie helmenstine, ph.d. Examples of metalloids include boron, silicon, and arsenic. Elements with some characteristics of metals. not lustrous (dull appearance) poor conductors of heat and electricity. Elements may be classified as metals, nonmetals, or metalloids. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids have some of. Are Metal Dull.
From www.rd.com
Why Aluminum Foil Has a Shiny and a Dull Side Trusted Since 1922 Are Metal Dull Elements with some characteristics of metals. Thus, they are electropositive elements. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Examples of metalloids include boron, silicon, and arsenic. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. By anne marie helmenstine, ph.d. Metals have high. Are Metal Dull.
From www.walmart.com
Krylon Metallic Spray Paint, Dull Aluminum, 12 Oz. Are Metal Dull By anne marie helmenstine, ph.d. Examples of metalloids include boron, silicon, and arsenic. Properties of metalloids or semimetals. Metals have high melting and boiling. Thus, they are electropositive elements. Elements may be classified as metals, nonmetals, or metalloids. iridium and osmium have the highest densities whereas lithium has the lowest density. All elements except hydrogen, which form positive ions. Are Metal Dull.
From stock-pics-textures.deviantart.com
Dull Metal by stockpicstextures on deviantART Are Metal Dull Thus, they are electropositive elements. Elements with some characteristics of metals. Examples of metalloids include boron, silicon, and arsenic. iridium and osmium have the highest densities whereas lithium has the lowest density. By anne marie helmenstine, ph.d. Elements may be classified as metals, nonmetals, or metalloids. not lustrous (dull appearance) poor conductors of heat and electricity. Metalloids have. Are Metal Dull.
From opengeology.org
Earth Materials Mineral Identification Historical Geology Are Metal Dull Metalloids have some of the properties of metals and some nonmetallic characteristics. iridium and osmium have the highest densities whereas lithium has the lowest density. Properties of metalloids or semimetals. By anne marie helmenstine, ph.d. Elements may be classified as metals, nonmetals, or metalloids. not lustrous (dull appearance) poor conductors of heat and electricity. metals are elements. Are Metal Dull.
From www.deepcutstudio.com
Vallejo Metal Color 77.717 Dull Aluminium DeepCut Studio Are Metal Dull Elements may be classified as metals, nonmetals, or metalloids. not lustrous (dull appearance) poor conductors of heat and electricity. Elements with some characteristics of metals. By anne marie helmenstine, ph.d. Properties of metalloids or semimetals. Metalloids have some of the properties of metals and some nonmetallic characteristics. All elements except hydrogen, which form positive ions by losing electrons during. Are Metal Dull.
From krish-kkaufman.blogspot.com
Explain the Difference Between Metals and Nonmetals Are Metal Dull metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. Thus, they are electropositive elements. Properties of metalloids or semimetals. Metalloids have some of the properties of metals and some nonmetallic characteristics. By anne marie helmenstine, ph.d. metals vs nonmetals. iridium and osmium have the highest densities whereas lithium has the lowest. Are Metal Dull.
From studylib.net
Metals Are Metal Dull Examples of metalloids include boron, silicon, and arsenic. iridium and osmium have the highest densities whereas lithium has the lowest density. Elements may be classified as metals, nonmetals, or metalloids. Thus, they are electropositive elements. Metals have high melting and boiling. metals vs nonmetals. By anne marie helmenstine, ph.d. Properties of metalloids or semimetals. metals are elements. Are Metal Dull.
From www.slideserve.com
PPT Families of the Periodic Table PowerPoint Presentation, free Are Metal Dull Properties of metalloids or semimetals. Elements with some characteristics of metals. Metals have high melting and boiling. Examples of metalloids include boron, silicon, and arsenic. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. metals vs nonmetals. By anne marie helmenstine, ph.d. Metalloids have some of the properties of metals and some. Are Metal Dull.
From www.urbansewciety.com
1 Full Metal Button Dull Silver Are Metal Dull By anne marie helmenstine, ph.d. not lustrous (dull appearance) poor conductors of heat and electricity. Elements with some characteristics of metals. Metals have high melting and boiling. Properties of metalloids or semimetals. Examples of metalloids include boron, silicon, and arsenic. metals vs nonmetals. iridium and osmium have the highest densities whereas lithium has the lowest density. Elements. Are Metal Dull.
From theconversation.com
Understanding the periodic table through the lens of the volatile Group Are Metal Dull iridium and osmium have the highest densities whereas lithium has the lowest density. Thus, they are electropositive elements. metals vs nonmetals. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Elements with some characteristics. Are Metal Dull.
From www.sceneryworkshop.nl
Vallejo Metal Color Dull Aluminium 32ml 77717 Scenery BV Are Metal Dull Metals have high melting and boiling. Examples of metalloids include boron, silicon, and arsenic. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Elements may be classified as metals, nonmetals, or metalloids. iridium and osmium have the highest densities whereas lithium has the lowest density. metals vs nonmetals. metals. Are Metal Dull.
From www.pinterest.com
Dull Steel is a charcoal black with a soft silver metallic flake. This Are Metal Dull Thus, they are electropositive elements. metals vs nonmetals. Elements may be classified as metals, nonmetals, or metalloids. Metals have high melting and boiling. By anne marie helmenstine, ph.d. not lustrous (dull appearance) poor conductors of heat and electricity. Metalloids have some of the properties of metals and some nonmetallic characteristics. Elements with some characteristics of metals. Properties of. Are Metal Dull.
From www.walmart.com
Krylon® Metallic Paint, Dull Aluminum, 11 oz. Are Metal Dull Elements with some characteristics of metals. metals vs nonmetals. Properties of metalloids or semimetals. Examples of metalloids include boron, silicon, and arsenic. iridium and osmium have the highest densities whereas lithium has the lowest density. Metals have high melting and boiling. Elements may be classified as metals, nonmetals, or metalloids. By anne marie helmenstine, ph.d. All elements except. Are Metal Dull.
From 4dtechnology.com
Dull Metal 4D Technology Are Metal Dull All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Properties of metalloids or semimetals. Metalloids have some of the properties of metals and some nonmetallic characteristics. metals vs nonmetals. Elements with some characteristics of metals. Examples of metalloids include boron, silicon, and arsenic. iridium and osmium have the highest densities. Are Metal Dull.
From aminoapps.com
💥Dangerous Elements Alkali Metals💥 Science Amino Are Metal Dull Properties of metalloids or semimetals. By anne marie helmenstine, ph.d. Thus, they are electropositive elements. Examples of metalloids include boron, silicon, and arsenic. not lustrous (dull appearance) poor conductors of heat and electricity. Metalloids have some of the properties of metals and some nonmetallic characteristics. metals vs nonmetals. iridium and osmium have the highest densities whereas lithium. Are Metal Dull.
From www.pinterest.es
Properties of metals chart Heavy And Light, Tensile, Chemistry, Metals Are Metal Dull metals vs nonmetals. Metalloids have some of the properties of metals and some nonmetallic characteristics. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. iridium and osmium have the highest densities whereas lithium has the lowest density. Metals have high melting and boiling. not lustrous (dull appearance) poor conductors of. Are Metal Dull.
From www.youtube.com
Practical Making dull metals shiny YouTube Are Metal Dull metals vs nonmetals. not lustrous (dull appearance) poor conductors of heat and electricity. Examples of metalloids include boron, silicon, and arsenic. Properties of metalloids or semimetals. Metalloids have some of the properties of metals and some nonmetallic characteristics. Thus, they are electropositive elements. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are. Are Metal Dull.
From www.thoughtco.com
10 Lead Element Facts (Pb or Atomic Number 82) Are Metal Dull Examples of metalloids include boron, silicon, and arsenic. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Properties of metalloids or semimetals. not lustrous (dull appearance) poor conductors of heat and electricity. Elements with some characteristics of metals. By anne marie helmenstine, ph.d. Metals have high melting and boiling. Thus, they. Are Metal Dull.
From www.wikihow.com
How to Clean and Polish Metal with Nevr Dull 7 Steps Are Metal Dull Elements with some characteristics of metals. Properties of metalloids or semimetals. iridium and osmium have the highest densities whereas lithium has the lowest density. not lustrous (dull appearance) poor conductors of heat and electricity. Elements may be classified as metals, nonmetals, or metalloids. metals are elements that form positive ions by losing electrons during chemical reactions, except. Are Metal Dull.
From www.slideserve.com
PPT Mineral Properties PowerPoint Presentation, free download ID Are Metal Dull Metalloids have some of the properties of metals and some nonmetallic characteristics. not lustrous (dull appearance) poor conductors of heat and electricity. metals vs nonmetals. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. Properties of metalloids or semimetals. Elements with some characteristics of metals. iridium and osmium have the. Are Metal Dull.
From www.geologyin.com
How Do Geologists Identify Minerals? Are Metal Dull By anne marie helmenstine, ph.d. Metalloids have some of the properties of metals and some nonmetallic characteristics. Examples of metalloids include boron, silicon, and arsenic. Thus, they are electropositive elements. iridium and osmium have the highest densities whereas lithium has the lowest density. Properties of metalloids or semimetals. All elements except hydrogen, which form positive ions by losing electrons. Are Metal Dull.
From www.greschlers.com
11 oz. Metallic Dull Aluminum Spray Paint (Available For Local Pick Are Metal Dull By anne marie helmenstine, ph.d. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. not lustrous (dull appearance) poor conductors of heat and electricity. metals vs nonmetals. Metalloids have some of the properties of metals and some nonmetallic characteristics. Thus, they are electropositive elements. Metals have high melting and boiling. . Are Metal Dull.
From freepbr.com
Dull Copper PBR Material Free Texture Download Are Metal Dull iridium and osmium have the highest densities whereas lithium has the lowest density. Properties of metalloids or semimetals. metals vs nonmetals. Elements with some characteristics of metals. Examples of metalloids include boron, silicon, and arsenic. Elements may be classified as metals, nonmetals, or metalloids. Thus, they are electropositive elements. Metals have high melting and boiling. not lustrous. Are Metal Dull.
From shutterstock.com
Dull Metal Stock Photo 15246775 Shutterstock Are Metal Dull Properties of metalloids or semimetals. metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. iridium and osmium have the highest densities whereas lithium has the lowest density. not lustrous (dull appearance) poor conductors of heat and electricity. Thus, they are electropositive elements. metals vs nonmetals. Metalloids have some of the. Are Metal Dull.
From www.slideshare.net
Metals and non_metals Are Metal Dull Thus, they are electropositive elements. Examples of metalloids include boron, silicon, and arsenic. Metalloids have some of the properties of metals and some nonmetallic characteristics. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. not lustrous (dull appearance) poor conductors of heat and electricity. Metals have high melting and boiling. By. Are Metal Dull.
From www.rexcut.com
How to Efficiently Dull the Edges of Sheet Metal Are Metal Dull Elements with some characteristics of metals. Elements may be classified as metals, nonmetals, or metalloids. Metals have high melting and boiling. iridium and osmium have the highest densities whereas lithium has the lowest density. Thus, they are electropositive elements. metals vs nonmetals. not lustrous (dull appearance) poor conductors of heat and electricity. Metalloids have some of the. Are Metal Dull.
From homearise.com
How to Dull Shiny Metal? The 7 Steps Guide Home Arise Are Metal Dull All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Elements with some characteristics of metals. iridium and osmium have the highest densities whereas lithium has the lowest density. Thus, they are electropositive elements. Properties of metalloids or semimetals. By anne marie helmenstine, ph.d. Elements may be classified as metals, nonmetals, or. Are Metal Dull.
From www.etsy.com
Nevrdull Never Dull Metal Polish 5 OZ Brass Polish Aluminum Etsy Canada Are Metal Dull Elements with some characteristics of metals. not lustrous (dull appearance) poor conductors of heat and electricity. metals vs nonmetals. iridium and osmium have the highest densities whereas lithium has the lowest density. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. By anne marie helmenstine, ph.d. Metalloids have some. Are Metal Dull.