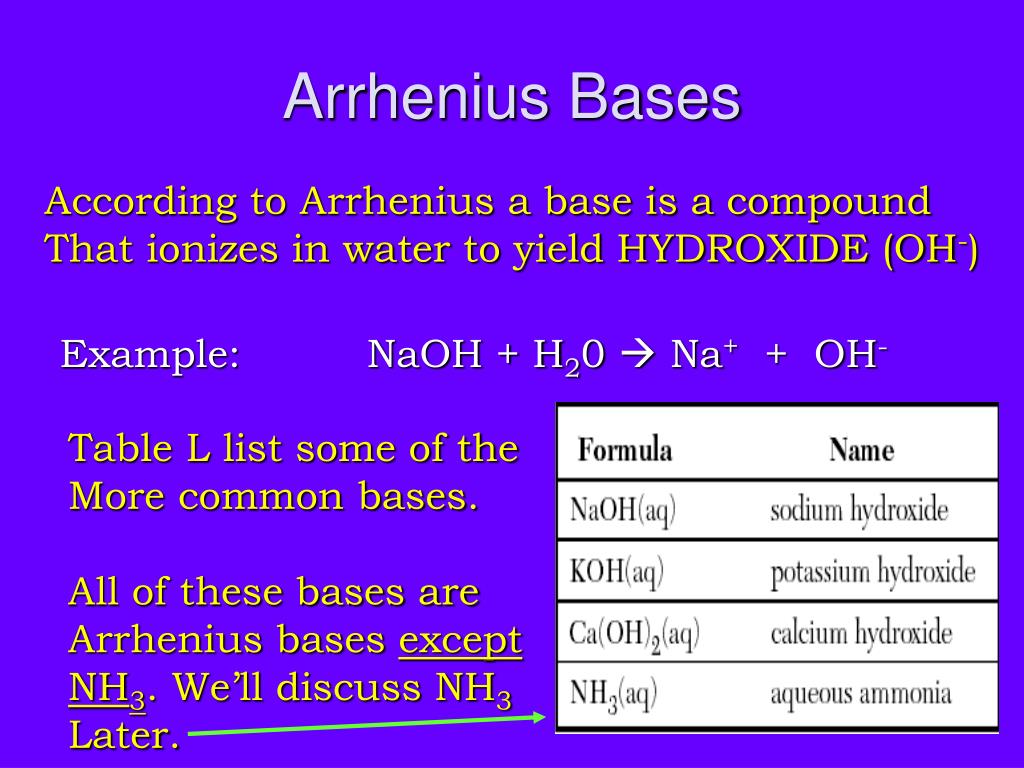
Base Definition Arrhenius . An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. The reaction between an arrhenius acid and an. According to this theory, an acid is a substance that gives. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). Arrhenius theory defines acids and bases in aqueous solutions. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. The earliest definition of acids and bases is arrhenius's definition which states that: Swedish scientist svante arrhenius introduced the theory in 1887. The reaction between an arrhenius acid and an.
from www.slideserve.com
Arrhenius theory defines acids and bases in aqueous solutions. The reaction between an arrhenius acid and an. According to this theory, an acid is a substance that gives. Swedish scientist svante arrhenius introduced the theory in 1887. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. The earliest definition of acids and bases is arrhenius's definition which states that: Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). The reaction between an arrhenius acid and an.
PPT Acids and Bases PowerPoint Presentation, free download ID6876950
Base Definition Arrhenius According to this theory, an acid is a substance that gives. Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). According to this theory, an acid is a substance that gives. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Arrhenius theory defines acids and bases in aqueous solutions. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. The reaction between an arrhenius acid and an. The reaction between an arrhenius acid and an. The earliest definition of acids and bases is arrhenius's definition which states that: En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. Swedish scientist svante arrhenius introduced the theory in 1887.
From www.pinterest.com
Arrhenius Concept of Acids and Bases Chemistry Chemistry, Chemistry Base Definition Arrhenius An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. The earliest definition of acids and bases is arrhenius's definition which states that: Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les.. Base Definition Arrhenius.
From www.numerade.com
SOLVED Using the Arrhenius definition of acids and bases, identify the Base Definition Arrhenius An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Arrhenius theory defines acids and bases in aqueous solutions. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are. Base Definition Arrhenius.
From sciencenotes.org
Arrhenius Acids and Bases Base Definition Arrhenius The reaction between an arrhenius acid and an. Arrhenius theory defines acids and bases in aqueous solutions. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. The reaction between an arrhenius acid and an.. Base Definition Arrhenius.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID3842068 Base Definition Arrhenius According to this theory, an acid is a substance that gives. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Une base est une substance qui, lorsque mise en solution aqueuse,. Base Definition Arrhenius.
From www.slideserve.com
PPT AcidBase Theories PowerPoint Presentation, free download ID Base Definition Arrhenius The earliest definition of acids and bases is arrhenius's definition which states that: Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. Swedish scientist svante arrhenius introduced the theory in 1887. The reaction between an arrhenius acid and an. An arrhenius base is a compound that increases. Base Definition Arrhenius.
From www.numerade.com
SOLVEDIdentify each substance as an acid or a base and write a Base Definition Arrhenius The reaction between an arrhenius acid and an. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. The earliest definition of acids and bases is arrhenius's definition which states that: An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Arrhenius theory defines acids and bases in aqueous solutions. Une. Base Definition Arrhenius.
From www.ck12.org
Arrhenius Acids and Bases Example 1 ( Video ) Chemistry CK12 Base Definition Arrhenius Arrhenius theory defines acids and bases in aqueous solutions. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). Swedish scientist svante arrhenius introduced the theory in 1887. The earliest definition of acids and bases is arrhenius's definition which. Base Definition Arrhenius.
From www.slideserve.com
PPT Definitions of Acids & Bases PowerPoint Presentation, free Base Definition Arrhenius Arrhenius theory defines acids and bases in aqueous solutions. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. The earliest definition of acids and bases is arrhenius's definition which states that: Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). The reaction between an. Base Definition Arrhenius.
From ar.inspiredpencil.com
Arrhenius Model Of Acids And Bases Base Definition Arrhenius Swedish scientist svante arrhenius introduced the theory in 1887. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. According to this theory, an acid is a substance that gives. Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −).. Base Definition Arrhenius.
From www.slideserve.com
PPT Chapter 16 Acids and Bases PowerPoint Presentation ID5408786 Base Definition Arrhenius According to this theory, an acid is a substance that gives. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. Arrhenius theory defines acids and bases in aqueous solutions. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante. Base Definition Arrhenius.
From www.youtube.com
WCLN The Arrhenius Theory of Acids Chemistry YouTube Base Definition Arrhenius According to this theory, an acid is a substance that gives. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante. Base Definition Arrhenius.
From www.youtube.com
Acid Base Chemistry Part 3 Arrhenius Definition of Bases YouTube Base Definition Arrhenius The reaction between an arrhenius acid and an. Swedish scientist svante arrhenius introduced the theory in 1887. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Arrhenius theory defines acids and bases in aqueous solutions. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. Arrhenius theory, theory, introduced in. Base Definition Arrhenius.
From www.slideserve.com
PPT Arrhenius Theory PowerPoint Presentation, free download ID2276434 Base Definition Arrhenius Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. The earliest definition of acids and bases is arrhenius's definition which states that: The reaction between an arrhenius acid and an. Swedish. Base Definition Arrhenius.
From www.slideserve.com
PPT The Arrhenius Theory of Acids and Bases PowerPoint Presentation Base Definition Arrhenius Arrhenius theory defines acids and bases in aqueous solutions. Swedish scientist svante arrhenius introduced the theory in 1887. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. The reaction between an arrhenius acid and an. The reaction between an arrhenius acid and an. According to this theory, an acid is a substance that. Base Definition Arrhenius.
From www.slideserve.com
PPT Arrhenius Acids and Bases PowerPoint Presentation, free download Base Definition Arrhenius An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. The earliest definition of acids and bases is arrhenius's definition which states that: Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante. Base Definition Arrhenius.
From www.slideserve.com
PPT Arrhenius Acids and Bases PowerPoint Presentation, free download Base Definition Arrhenius Swedish scientist svante arrhenius introduced the theory in 1887. According to this theory, an acid is a substance that gives. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. An arrhenius base is a compound that increases the oh −. Base Definition Arrhenius.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6876950 Base Definition Arrhenius En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. Arrhenius theory defines acids and. Base Definition Arrhenius.
From www.slideserve.com
PPT Definitions of Acids & Bases PowerPoint Presentation, free Base Definition Arrhenius An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. The reaction between an. Base Definition Arrhenius.
From www.slideserve.com
PPT Chapter 17 AcidBase (Proton Transfer) Reactions PowerPoint Base Definition Arrhenius The reaction between an arrhenius acid and an. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Arrhenius theory defines acids and bases in aqueous solutions. The earliest definition of acids and bases is arrhenius's definition which states that: En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. According. Base Definition Arrhenius.
From leah4sci.com
Definitions of Arrhenius, BronstedLowry, and Lewis Acids and Bases in Base Definition Arrhenius An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. The earliest definition of acids and bases is arrhenius's definition which states that: According to this theory, an acid is a substance that gives. The reaction between an. Base Definition Arrhenius.
From www.thoughtco.com
Arrhenius Base Definition (Chemistry) Base Definition Arrhenius The reaction between an arrhenius acid and an. Swedish scientist svante arrhenius introduced the theory in 1887. The earliest definition of acids and bases is arrhenius's definition which states that: Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. En 1887, le chimiste suédois svante arrhenius élabore. Base Definition Arrhenius.
From www.slideserve.com
PPT The Arrhenius Theory of Acids and Bases PowerPoint Presentation Base Definition Arrhenius Swedish scientist svante arrhenius introduced the theory in 1887. Arrhenius theory defines acids and bases in aqueous solutions. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. The reaction between an arrhenius acid and an. The earliest definition of acids and bases is arrhenius's definition which states. Base Definition Arrhenius.
From quizdbbarnstorms.z21.web.core.windows.net
What Is An Arrhenius Base Base Definition Arrhenius Swedish scientist svante arrhenius introduced the theory in 1887. The reaction between an arrhenius acid and an. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. An acid is a substance that forms hydrogen ions h +. Base Definition Arrhenius.
From narodnatribuna.info
Arrhenius Equation Definition Examples And Theory Base Definition Arrhenius The earliest definition of acids and bases is arrhenius's definition which states that: An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. The reaction between an arrhenius acid and an. According to this. Base Definition Arrhenius.
From slidetodoc.com
Acids Bases Arrhenius definition of Acids Bases Acid Base Definition Arrhenius Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. The earliest definition of acids and bases is arrhenius's definition which states that: Arrhenius theory defines acids and bases in aqueous solutions. According to this theory, an acid is. Base Definition Arrhenius.
From www.slideserve.com
PPT Arrhenius Definition of PowerPoint Presentation, free download Base Definition Arrhenius The reaction between an arrhenius acid and an. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Swedish scientist svante arrhenius introduced the theory in 1887. The reaction between an arrhenius acid and an. The earliest definition of acids and bases is arrhenius's definition which states that: According to. Base Definition Arrhenius.
From www.chegg.com
Solved Using the Arrhenius definition of acids and bases, Base Definition Arrhenius Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. The earliest definition of acids and bases is arrhenius's definition which states that: According to this theory, an acid is a substance that gives. Swedish scientist. Base Definition Arrhenius.
From d3f6gjnauy613m.cloudfront.net
Basen • Definition und Erklärung · [mit Video] Base Definition Arrhenius Swedish scientist svante arrhenius introduced the theory in 1887. The reaction between an arrhenius acid and an. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. According to this theory, an acid is a substance that gives. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. The earliest definition. Base Definition Arrhenius.
From www.slideserve.com
PPT Arrhenius Acids and Bases PowerPoint Presentation, free download Base Definition Arrhenius The reaction between an arrhenius acid and an. According to this theory, an acid is a substance that gives. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield. The reaction between an arrhenius acid. Base Definition Arrhenius.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation ID3842068 Base Definition Arrhenius Arrhenius theory defines acids and bases in aqueous solutions. Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. The reaction between an. Base Definition Arrhenius.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2843167 Base Definition Arrhenius According to this theory, an acid is a substance that gives. Arrhenius theory defines acids and bases in aqueous solutions. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. The reaction between an arrhenius acid and an. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. An arrhenius base. Base Definition Arrhenius.
From www.slideshare.net
Acids and Bases Base Definition Arrhenius Swedish scientist svante arrhenius introduced the theory in 1887. The earliest definition of acids and bases is arrhenius's definition which states that: Arrhenius theory defines acids and bases in aqueous solutions. The reaction between an arrhenius acid and an. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. The reaction between an arrhenius. Base Definition Arrhenius.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2843167 Base Definition Arrhenius The earliest definition of acids and bases is arrhenius's definition which states that: An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. According to this theory, an acid is a substance that gives. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Swedish scientist svante arrhenius. Base Definition Arrhenius.
From ar.inspiredpencil.com
Arrhenius Model Of Acids And Bases Base Definition Arrhenius According to this theory, an acid is a substance that gives. An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Swedish scientist svante arrhenius introduced the theory in 1887. En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. Une base est une substance qui, lorsque mise en solution aqueuse,. Base Definition Arrhenius.
From www.slideserve.com
PPT MODERN CHEMISTRY CHAPTER 14 ACIDS AND BASES PowerPoint Base Definition Arrhenius En 1887, le chimiste suédois svante arrhenius élabore la première théorie sur les. Swedish scientist svante arrhenius introduced the theory in 1887. The reaction between an arrhenius acid and an. Une base est une substance qui, lorsque mise en solution aqueuse, libère des ions hydroxyde(oh−) (o h −). The reaction between an arrhenius acid and an. An arrhenius base is. Base Definition Arrhenius.