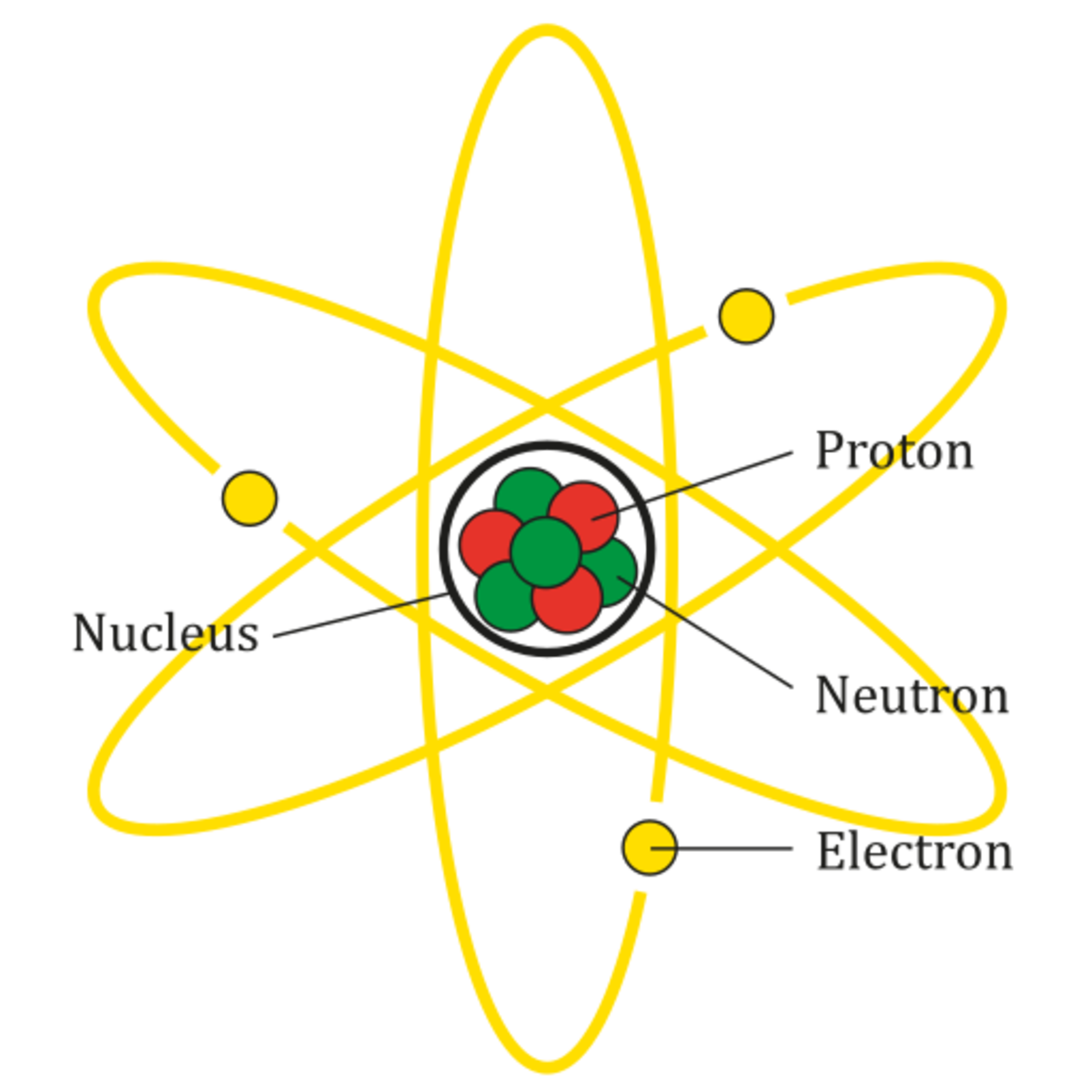
Why Do Atoms Don't Have A Charge . Because neutrons are not charged, they don't affect the overall charge of the atom. What is the reason why atoms have no electric charge? The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. Since an atom contains equal number of protons and electrons, it has no overall charge: Explain why an atom has no overall charge. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Protons have a +1 charge, and. The northern lights aren’t caused by atoms, because atoms are not. The number of protons in an atom is equal to the number of. An atom has no overall charge because each element has the same number of protons and electrons. But sometimes an atom can gain or lose an electron to become what's called an ion. An atom is comprised of protons, neutrons, and electrons. An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. Protons have positive charge and the.
from classfullplymouth.z21.web.core.windows.net
Because neutrons are not charged, they don't affect the overall charge of the atom. Protons have a +1 charge, and. What is the reason why atoms have no electric charge? An atom has no overall charge because each element has the same number of protons and electrons. The number of protons in an atom is equal to the number of. The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. The northern lights aren’t caused by atoms, because atoms are not. Since an atom contains equal number of protons and electrons, it has no overall charge: An atom is comprised of protons, neutrons, and electrons.
Parts Of The Atom
Why Do Atoms Don't Have A Charge Explain why an atom has no overall charge. The northern lights aren’t caused by atoms, because atoms are not. An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. Because neutrons are not charged, they don't affect the overall charge of the atom. Explain why an atom has no overall charge. An atom is comprised of protons, neutrons, and electrons. The number of protons in an atom is equal to the number of. An atom has no overall charge because each element has the same number of protons and electrons. Protons have positive charge and the. Since an atom contains equal number of protons and electrons, it has no overall charge: The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Protons have a +1 charge, and. But sometimes an atom can gain or lose an electron to become what's called an ion. The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. What is the reason why atoms have no electric charge?
From www.thoughtco.com
Basic Model of the Atom Atomic Theory Why Do Atoms Don't Have A Charge Protons have positive charge and the. Explain why an atom has no overall charge. But sometimes an atom can gain or lose an electron to become what's called an ion. An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. Protons have a +1 charge, and. The. Why Do Atoms Don't Have A Charge.
From www.thesciencehive.co.uk
Atomic Structure* — the science sauce Why Do Atoms Don't Have A Charge An atom has no overall charge because each element has the same number of protons and electrons. Because neutrons are not charged, they don't affect the overall charge of the atom. Explain why an atom has no overall charge. The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. What is the. Why Do Atoms Don't Have A Charge.
From simpleeducation.biz
Why Do Atoms Bond? Simple Education Why Do Atoms Don't Have A Charge The northern lights aren’t caused by atoms, because atoms are not. Since an atom contains equal number of protons and electrons, it has no overall charge: The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. Protons have positive charge and the. An atom has no overall electric charge because the number. Why Do Atoms Don't Have A Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Why Do Atoms Don't Have A Charge The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An atom is comprised of protons, neutrons, and electrons. What is the reason why atoms have no electric charge? An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons.. Why Do Atoms Don't Have A Charge.
From www.slideserve.com
PPT The Atom PowerPoint Presentation, free download ID5056341 Why Do Atoms Don't Have A Charge Explain why an atom has no overall charge. The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. An atom is comprised of protons, neutrons, and electrons. Because neutrons are not charged, they don't affect the overall charge of the atom. Protons have positive charge and the. An atom has no overall. Why Do Atoms Don't Have A Charge.
From classfullplymouth.z21.web.core.windows.net
Parts Of The Atom Why Do Atoms Don't Have A Charge The number of protons in an atom is equal to the number of. An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. The northern lights aren’t caused by atoms, because atoms are not. Because neutrons are not charged, they don't affect the overall charge of the. Why Do Atoms Don't Have A Charge.
From masterconceptsinchemistry.com
How hydrogen atoms share valence electrons to form covalent bond and Why Do Atoms Don't Have A Charge The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An atom has no overall charge because each element has the same number of protons and electrons. An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. An atom. Why Do Atoms Don't Have A Charge.
From sciencenotes.org
Learn the Parts of an Atom Why Do Atoms Don't Have A Charge Protons have a +1 charge, and. An atom is comprised of protons, neutrons, and electrons. The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. Explain why an atom has no overall charge. The northern lights aren’t caused by atoms, because atoms are not. An atom has no overall charge because each. Why Do Atoms Don't Have A Charge.
From www.slideserve.com
PPT Atoms have NO overall charge PowerPoint Presentation, free Why Do Atoms Don't Have A Charge But sometimes an atom can gain or lose an electron to become what's called an ion. An atom is comprised of protons, neutrons, and electrons. Protons have a +1 charge, and. The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. The positive charges on all the protons are exactly balanced by. Why Do Atoms Don't Have A Charge.
From www.worksheetsplanet.com
What is an Atom Meaning & Definition of Atom Why Do Atoms Don't Have A Charge The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. Protons have positive charge and the. Because neutrons are not charged, they don't affect the overall charge of the atom. The northern lights aren’t caused by atoms, because atoms are not. But sometimes an atom can gain or lose an electron to. Why Do Atoms Don't Have A Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Why Do Atoms Don't Have A Charge Because neutrons are not charged, they don't affect the overall charge of the atom. The northern lights aren’t caused by atoms, because atoms are not. An atom has no overall charge because each element has the same number of protons and electrons. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons.. Why Do Atoms Don't Have A Charge.
From www.slideserve.com
PPT Lecture 1 Charge and Coulomb’s Law PowerPoint Presentation, free Why Do Atoms Don't Have A Charge Since an atom contains equal number of protons and electrons, it has no overall charge: Explain why an atom has no overall charge. An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. Protons have a +1 charge, and. An atom is comprised of protons, neutrons, and. Why Do Atoms Don't Have A Charge.
From www.worksheetsplanet.com
Bohr's Atomic Model Why Do Atoms Don't Have A Charge The northern lights aren’t caused by atoms, because atoms are not. Protons have positive charge and the. The number of protons in an atom is equal to the number of. An atom is comprised of protons, neutrons, and electrons. Explain why an atom has no overall charge. The point is that electrons, fundamental particles of negligible mass, have an opposite. Why Do Atoms Don't Have A Charge.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together Why Do Atoms Don't Have A Charge An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. Protons have a +1 charge, and. But sometimes an atom can gain or lose an electron to become what's called an ion. Protons have positive charge and the. Because neutrons are not charged, they don't affect the. Why Do Atoms Don't Have A Charge.
From periodictable.me
What are the Difference Between Charge and Electron? Dynamic Periodic Why Do Atoms Don't Have A Charge What is the reason why atoms have no electric charge? An atom is comprised of protons, neutrons, and electrons. But sometimes an atom can gain or lose an electron to become what's called an ion. Since an atom contains equal number of protons and electrons, it has no overall charge: Protons have a +1 charge, and. Explain why an atom. Why Do Atoms Don't Have A Charge.
From spmscience.blog.onlinetuition.com.my
4.2 Structure of Atoms SPM Science Why Do Atoms Don't Have A Charge An atom is comprised of protons, neutrons, and electrons. Protons have positive charge and the. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. Protons have a +1 charge, and. An atom has. Why Do Atoms Don't Have A Charge.
From www.livescience.com
What Is an Atom? Live Science Why Do Atoms Don't Have A Charge An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. Protons have positive charge and the. But sometimes an atom can gain or lose an electron to become what's called an ion. What is the reason why atoms have no electric charge? An atom is comprised of. Why Do Atoms Don't Have A Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Why Do Atoms Don't Have A Charge An atom is comprised of protons, neutrons, and electrons. An atom has no overall charge because each element has the same number of protons and electrons. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to. Why Do Atoms Don't Have A Charge.
From sciencenotes.org
Octet Rule Definition, Examples, and Exceptions Why Do Atoms Don't Have A Charge Explain why an atom has no overall charge. The northern lights aren’t caused by atoms, because atoms are not. An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. Because neutrons are not charged, they don't affect the overall charge of the atom. Protons have positive charge. Why Do Atoms Don't Have A Charge.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Why Do Atoms Don't Have A Charge What is the reason why atoms have no electric charge? An atom has no overall charge because each element has the same number of protons and electrons. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Protons have a +1 charge, and. Because neutrons are not charged, they don't affect the. Why Do Atoms Don't Have A Charge.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Why Do Atoms Don't Have A Charge But sometimes an atom can gain or lose an electron to become what's called an ion. An atom is comprised of protons, neutrons, and electrons. Explain why an atom has no overall charge. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. Protons have positive charge and the. An atom has. Why Do Atoms Don't Have A Charge.
From depositphotos.com
What Happens Atoms Bond Infographic Diagram Showing How Electrons Why Do Atoms Don't Have A Charge But sometimes an atom can gain or lose an electron to become what's called an ion. The number of protons in an atom is equal to the number of. An atom has no overall charge because each element has the same number of protons and electrons. The point is that electrons, fundamental particles of negligible mass, have an opposite electric. Why Do Atoms Don't Have A Charge.
From infraredforhealth.com
Which One Has No Charge? Infrared for Health Why Do Atoms Don't Have A Charge Because neutrons are not charged, they don't affect the overall charge of the atom. An atom has no overall charge because each element has the same number of protons and electrons. Protons have a +1 charge, and. Protons have positive charge and the. But sometimes an atom can gain or lose an electron to become what's called an ion. An. Why Do Atoms Don't Have A Charge.
From sciencenotes.org
10 Interesting Atom Facts Why Do Atoms Don't Have A Charge An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. The northern lights aren’t caused by atoms, because atoms are not. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An atom has no overall charge because each. Why Do Atoms Don't Have A Charge.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Why Do Atoms Don't Have A Charge An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. What is the reason why atoms have no electric charge? An atom is comprised of protons, neutrons, and electrons. Explain why an atom has no overall charge. The positive charges on all the protons are exactly balanced. Why Do Atoms Don't Have A Charge.
From www.nagwa.com
Question Video Explaining Why Atoms Do Not Have a Charge Nagwa Why Do Atoms Don't Have A Charge The northern lights aren’t caused by atoms, because atoms are not. But sometimes an atom can gain or lose an electron to become what's called an ion. The number of protons in an atom is equal to the number of. Protons have positive charge and the. Since an atom contains equal number of protons and electrons, it has no overall. Why Do Atoms Don't Have A Charge.
From sciencenotes.org
Element Charges Chart How to Know the Charge of an Atom Why Do Atoms Don't Have A Charge The northern lights aren’t caused by atoms, because atoms are not. An atom has no overall charge because each element has the same number of protons and electrons. Protons have a +1 charge, and. An atom is comprised of protons, neutrons, and electrons. The number of protons in an atom is equal to the number of. Because neutrons are not. Why Do Atoms Don't Have A Charge.
From www.sciencefacts.net
Atomic Nucleus Definition, Structure & Parts with Diagram Why Do Atoms Don't Have A Charge The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An atom is comprised of protons, neutrons, and electrons. Protons have a +1 charge, and. Explain why an atom has no overall charge. Because neutrons are not charged, they don't affect the overall charge of the atom. An atom has no overall. Why Do Atoms Don't Have A Charge.
From www.mindomo.com
Energy Mind Map Why Do Atoms Don't Have A Charge An atom is comprised of protons, neutrons, and electrons. The northern lights aren’t caused by atoms, because atoms are not. Protons have a +1 charge, and. An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. An atom has no overall charge because each element has the. Why Do Atoms Don't Have A Charge.
From www.science-sparks.com
A Brief History of the Atom Why Do Atoms Don't Have A Charge An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. Explain why an atom has no overall charge. The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. Because neutrons are not charged, they don't affect the overall charge. Why Do Atoms Don't Have A Charge.
From www.slideserve.com
PPT Types of Chemical Bonds PowerPoint Presentation, free download Why Do Atoms Don't Have A Charge The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An atom has no overall charge because each element has the same number of protons and electrons. An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. Protons have. Why Do Atoms Don't Have A Charge.
From www.broadlearnings.com
Atomic Structure Broad Learnings Why Do Atoms Don't Have A Charge What is the reason why atoms have no electric charge? An atom is comprised of protons, neutrons, and electrons. But sometimes an atom can gain or lose an electron to become what's called an ion. Protons have a +1 charge, and. The number of protons in an atom is equal to the number of. The positive charges on all the. Why Do Atoms Don't Have A Charge.
From circuitpennhockey01yh.z13.web.core.windows.net
Atom With Electrical Charge Why Do Atoms Don't Have A Charge An atom has no overall electric charge because the number of protons (which are positively charged) is equal to the number of electrons. The point is that electrons, fundamental particles of negligible mass, have an opposite electric charge to protons, massive. The positive charges on all the protons are exactly balanced by the negative charges on all the electrons. An. Why Do Atoms Don't Have A Charge.
From slides.com
Chemistry Why Do Atoms Don't Have A Charge But sometimes an atom can gain or lose an electron to become what's called an ion. The number of protons in an atom is equal to the number of. Explain why an atom has no overall charge. An atom has no overall charge because each element has the same number of protons and electrons. The point is that electrons, fundamental. Why Do Atoms Don't Have A Charge.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview Why Do Atoms Don't Have A Charge Because neutrons are not charged, they don't affect the overall charge of the atom. What is the reason why atoms have no electric charge? The northern lights aren’t caused by atoms, because atoms are not. Explain why an atom has no overall charge. An atom has no overall charge because each element has the same number of protons and electrons.. Why Do Atoms Don't Have A Charge.