Catalyst To A Chemical Reaction Results In . They do not appear in the reaction’s net equation and are not consumed during the reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. catalysts participate in a chemical reaction and increase its rate. catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
from www.alamy.com
catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; They do not appear in the reaction’s net. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
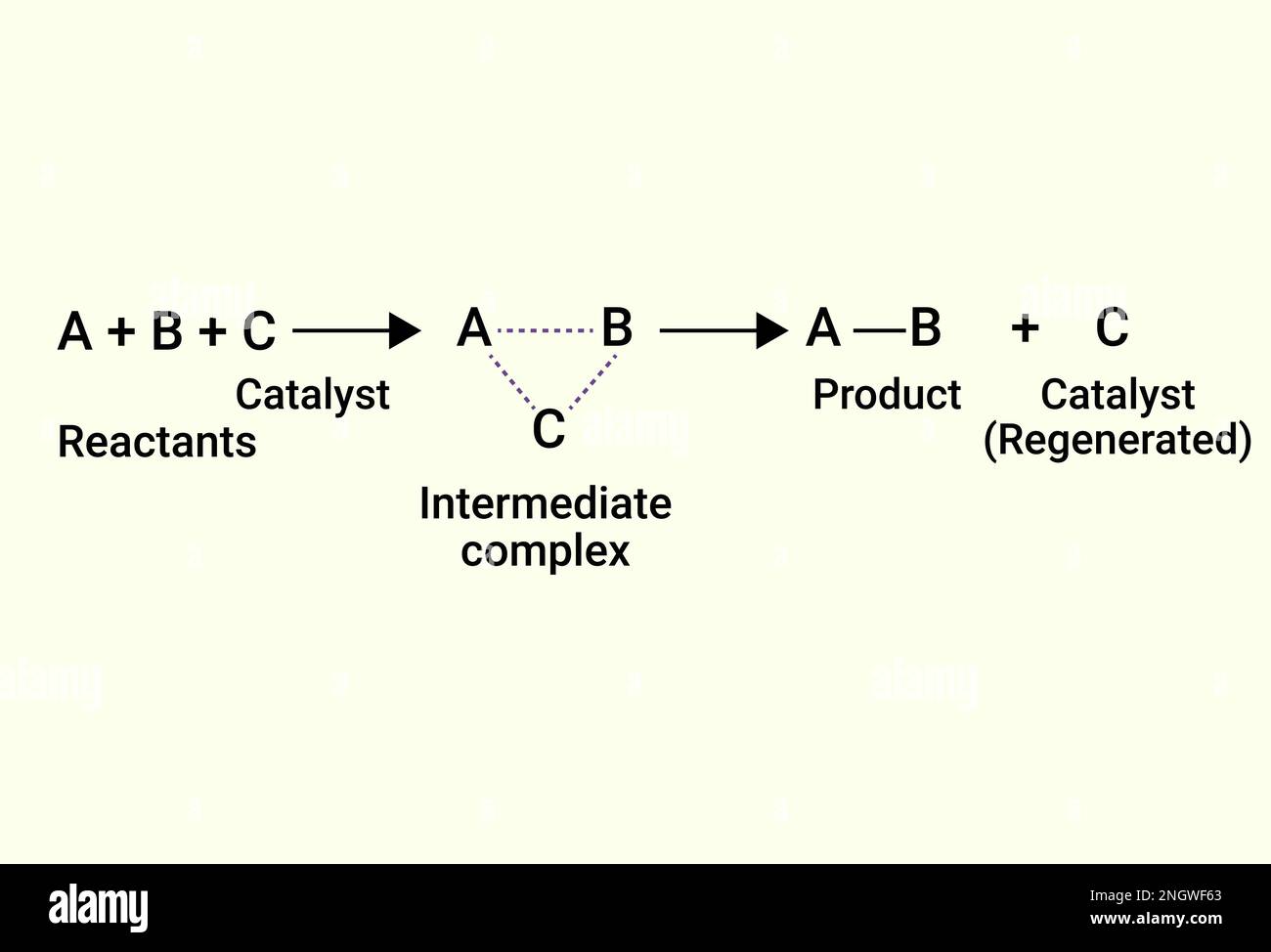
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy
Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. catalysts participate in a chemical reaction and increase its rate. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst To A Chemical Reaction Results In They do not appear in the reaction’s net. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts participate in a chemical reaction and increase its rate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts participate in a. Catalyst To A Chemical Reaction Results In.
From www.youtube.com
Practice Identifying Catalysts and Intermediates YouTube Catalyst To A Chemical Reaction Results In the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. They do not appear in the reaction’s net. catalysts affect the rate of a chemical reaction by altering its. Catalyst To A Chemical Reaction Results In.
From www.pinterest.com
Catalyst Easy Science in 2022 Energy activities, Chemical reactions Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts affect the rate of a chemical reaction by altering its mechanism to provide a. Catalyst To A Chemical Reaction Results In.
From fyobmixiq.blob.core.windows.net
Uses Of Catalysts In Chemical Reactions at Cecilia Keffer blog Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. catalysts participate in a chemical reaction and increase its rate. a catalyst binds to a reactant and it increases the number of collision between. Catalyst To A Chemical Reaction Results In.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst To A Chemical Reaction Results In catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts participate in a chemical reaction and increase its rate. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. catalysts affect the rate of a chemical reaction by. Catalyst To A Chemical Reaction Results In.
From www.essentialchemicalindustry.org
Catalysis in industry Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. They do not appear in the reaction’s net. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts. Catalyst To A Chemical Reaction Results In.
From www.nagwa.com
Question Video Identifying the Reason Why Catalysts Are Used in Catalyst To A Chemical Reaction Results In a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. catalysts participate in a chemical reaction and increase its rate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts participate in a chemical reaction. Catalyst To A Chemical Reaction Results In.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalyst To A Chemical Reaction Results In They do not appear in the reaction’s net. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts participate in a chemical reaction and increase its rate. a. Catalyst To A Chemical Reaction Results In.
From exyrpmmac.blob.core.windows.net
Catalysts Questions at Vera Little blog Catalyst To A Chemical Reaction Results In catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. catalysts. Catalyst To A Chemical Reaction Results In.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst To A Chemical Reaction Results In catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. They do not appear in the reaction’s net. catalysts participate in a chemical reaction and increase its rate. a. Catalyst To A Chemical Reaction Results In.
From ar.inspiredpencil.com
Catalyst Chemical Reaction Catalyst To A Chemical Reaction Results In They do not appear in the reaction’s net. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. catalysts participate in a chemical reaction and increase its. Catalyst To A Chemical Reaction Results In.
From cartoondealer.com
Enzyme As Catalyst In Chemical Reactions Vector Illustration Catalyst To A Chemical Reaction Results In the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. They do not appear in the reaction’s net equation and are not consumed during the reaction. catalyst, in chemistry, any. Catalyst To A Chemical Reaction Results In.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts participate in a chemical reaction and increase its. Catalyst To A Chemical Reaction Results In.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst To A Chemical Reaction Results In They do not appear in the reaction’s net equation and are not consumed during the reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts participate in a chemical reaction and increase its rate. catalysts participate in a chemical reaction and increase its rate. They do not appear in. Catalyst To A Chemical Reaction Results In.
From fyoerprzq.blob.core.windows.net
What Are Catalysts In Chemical Reactions at Brian Edwards blog Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is a. Catalyst To A Chemical Reaction Results In.
From www.slideserve.com
PPT Fast and Slow Chemistry PowerPoint Presentation, free download Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net. the most effective catalyst. Catalyst To A Chemical Reaction Results In.
From gioaralqv.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction In Such A Manner Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net. catalysts participate in a chemical reaction and increase its rate. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts affect the rate of a chemical reaction by altering its mechanism. Catalyst To A Chemical Reaction Results In.
From www.chegg.com
Solved Which of the following are true about catalysts and Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. catalysts participate in a chemical reaction and increase its. Catalyst To A Chemical Reaction Results In.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst To A Chemical Reaction Results In catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules,. Catalyst To A Chemical Reaction Results In.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst To A Chemical Reaction Results In the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; They do not appear in the reaction’s net equation and are not consumed during the reaction. catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. Catalyst To A Chemical Reaction Results In.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalyst To A Chemical Reaction Results In catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. . Catalyst To A Chemical Reaction Results In.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My Catalyst To A Chemical Reaction Results In the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts participate in a chemical reaction and increase its rate. catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. a catalyst is a. Catalyst To A Chemical Reaction Results In.
From giorbnkft.blob.core.windows.net
Catalyst Chemistry Term at Nancy Sherry blog Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; They do not appear in the reaction’s net equation and are not consumed during the reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. Catalyst To A Chemical Reaction Results In.
From www.slideserve.com
PPT The changes in energy that occur during chemical reactions can Catalyst To A Chemical Reaction Results In catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do not appear in the reaction’s net equation and are not consumed during the reaction. catalysts participate in a chemical reaction and increase its rate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a. Catalyst To A Chemical Reaction Results In.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Catalyst To A Chemical Reaction Results In They do not appear in the reaction’s net. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts participate in a chemical reaction and increase its rate. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for. Catalyst To A Chemical Reaction Results In.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst To A Chemical Reaction Results In catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts participate in a chemical reaction. Catalyst To A Chemical Reaction Results In.
From fyojpzftz.blob.core.windows.net
Does Catalyst Decreases The Rate Of Reaction at Eleanor Weaver blog Catalyst To A Chemical Reaction Results In a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. They do not appear in the reaction’s net. They do not appear in the reaction’s net equation and are not consumed during the reaction. catalyst, in chemistry, any substance that increases the rate of a reaction. Catalyst To A Chemical Reaction Results In.
From www.slideshare.net
Catalyst Catalyst To A Chemical Reaction Results In a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. catalysts participate in a chemical reaction and increase its rate. catalysts participate in a chemical reaction and increase its rate. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular. Catalyst To A Chemical Reaction Results In.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube Catalyst To A Chemical Reaction Results In a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. They do not appear in the reaction’s net equation and are not consumed during the reaction. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for. Catalyst To A Chemical Reaction Results In.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst To A Chemical Reaction Results In catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalyst, in chemistry, any substance that increases the rate of a reaction without. Catalyst To A Chemical Reaction Results In.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst To A Chemical Reaction Results In catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. They do not appear in the reaction’s net. catalysts participate in a chemical. Catalyst To A Chemical Reaction Results In.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalyst To A Chemical Reaction Results In They do not appear in the reaction’s net equation and are not consumed during the reaction. catalysts participate in a chemical reaction and increase its rate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst is a chemical substance that affects the rate of a chemical. Catalyst To A Chemical Reaction Results In.
From exycfsrlh.blob.core.windows.net
Enzymes Are Considered Reactants In The Chemical Reaction at Moses Love Catalyst To A Chemical Reaction Results In They do not appear in the reaction’s net. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net equation and are not consumed during the reaction. catalysts participate in a chemical reaction and increase its rate. the most effective catalyst of. Catalyst To A Chemical Reaction Results In.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Catalyst To A Chemical Reaction Results In catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. catalysts participate in a chemical reaction and increase its rate. catalysts affect the rate of a chemical reaction by. Catalyst To A Chemical Reaction Results In.
From fyoerprzq.blob.core.windows.net
What Are Catalysts In Chemical Reactions at Brian Edwards blog Catalyst To A Chemical Reaction Results In catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. They do not appear in the reaction’s net. catalyst, in chemistry, any substance that increases the rate. Catalyst To A Chemical Reaction Results In.