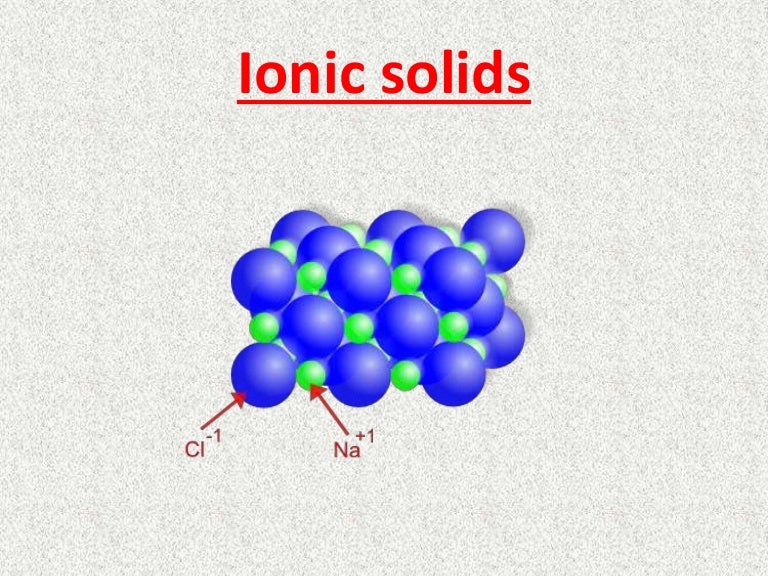
Are Ionic Solids Good Conductors . Ionic solids generally have high melting points and high boiling points. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic compounds have high melting points. Ionic compounds are hard and brittle. This is a result of the strong electrostatic. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). They have high melting and boiling points due to the strong ionic bonds. Ionic conductivity greatly varies between compounds. For some of them is much higher then for others. They are called fast ion.
from www.slideshare.net
Ionic compounds are hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. They are called fast ion. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). They have high melting and boiling points due to the strong ionic bonds. Ionic conductivity greatly varies between compounds. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. For some of them is much higher then for others. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. Ionic compounds have high melting points.
Ionic solids
Are Ionic Solids Good Conductors Ionic compounds have high melting points. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. They are called fast ion. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic conductivity greatly varies between compounds. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). For some of them is much higher then for others. This is a result of the strong electrostatic. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. Ionic compounds have high melting points. Ionic solids generally have high melting points and high boiling points. They have high melting and boiling points due to the strong ionic bonds. Ionic compounds are hard and brittle.
From studylib.net
Ionic conductors Are Ionic Solids Good Conductors Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. This is a result of the strong electrostatic. Ionic conductivity greatly varies between compounds. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. Solutions. Are Ionic Solids Good Conductors.
From onlinelibrary.wiley.com
Ionic Conductors Water‐Processable, Stretchable, Self‐Healable Are Ionic Solids Good Conductors Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. This is a result of the strong electrostatic. Ionic compounds are hard and brittle. Ionic compounds have high melting points. They have high melting and boiling points due to the strong ionic bonds. Ionic conductivity greatly varies between compounds. They are called. Are Ionic Solids Good Conductors.
From www.researchgate.net
A timeline of key development of Mg ion solid conductors (A) and a Are Ionic Solids Good Conductors For some of them is much higher then for others. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). This is a result of the strong electrostatic. Ionic conductivity greatly varies between compounds. They are called fast ion. Ionic compounds are hard and brittle. Ionic. Are Ionic Solids Good Conductors.
From www.researchgate.net
Ionic conductivity of proteinbased ion conductor (PIC) at room Are Ionic Solids Good Conductors Ionic solids generally have high melting points and high boiling points. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). Ionic compounds have high melting points. Ionic conductivity greatly varies between compounds. Ionic compounds do not conduct electricity as solids, but do conduct electricity when. Are Ionic Solids Good Conductors.
From www.science.org
Stretchable, Transparent, Ionic Conductors Science Are Ionic Solids Good Conductors They have high melting and boiling points due to the strong ionic bonds. For some of them is much higher then for others. Ionic conductivity greatly varies between compounds. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds are hard and brittle. Ionic solids generally have high melting points and high boiling points. Ionic. Are Ionic Solids Good Conductors.
From slideplayer.com
CHEMICAL BONDING. ppt download Are Ionic Solids Good Conductors Ionic solids generally have high melting points and high boiling points. This is a result of the strong electrostatic. Ionic compounds have high melting points. They are called fast ion. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). For some of them is much. Are Ionic Solids Good Conductors.
From www.doubtnut.com
Ionic solids are good conductors of electricity. Are Ionic Solids Good Conductors This is a result of the strong electrostatic. Ionic solids generally have high melting points and high boiling points. Ionic conductivity greatly varies between compounds. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. A variety of crystalline. Are Ionic Solids Good Conductors.
From brainly.in
difference between ionic solid and molecular solid..? Brainly.in Are Ionic Solids Good Conductors Ionic solids generally have high melting points and high boiling points. Ionic conductivity greatly varies between compounds. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions. Are Ionic Solids Good Conductors.
From slideplayer.com
Chemical Bonding Topic 5 Chemical Bonding Metallic Metal atoms only Are Ionic Solids Good Conductors Ionic compounds are hard and brittle. Ionic compounds have high melting points. Ionic solids generally have high melting points and high boiling points. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. They are called fast ion. For some of them is much higher then for others. Solutions of ionic compounds. Are Ionic Solids Good Conductors.
From www.w3schools.blog
Ionic Solids W3schools Are Ionic Solids Good Conductors Ionic compounds have high melting points. For some of them is much higher then for others. Ionic compounds are hard and brittle. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Solutions of ionic compounds and melted ionic compounds conduct electricity, but. Are Ionic Solids Good Conductors.
From slideplayer.com
Unit 6 Bonding How elements interact.. ppt download Are Ionic Solids Good Conductors This is a result of the strong electrostatic. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic conductivity greatly varies between compounds. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. Ionic. Are Ionic Solids Good Conductors.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Are Ionic Solids Good Conductors They are called fast ion. They have high melting and boiling points due to the strong ionic bonds. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds do not conduct. Are Ionic Solids Good Conductors.
From www.slideshare.net
Ionic solids Are Ionic Solids Good Conductors This is a result of the strong electrostatic. Ionic solids generally have high melting points and high boiling points. For some of them is much higher then for others. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution.. Are Ionic Solids Good Conductors.
From brainly.in
Q.1 Write an activity to show that ionic compounds are good conductors Are Ionic Solids Good Conductors They are called fast ion. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. They have high melting and boiling points due to the. Are Ionic Solids Good Conductors.
From getchemistry.weebly.com
Ionic Bonding Chemistry Are Ionic Solids Good Conductors Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. They are called fast ion. Ionic compounds have high melting points. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. A variety of crystalline phosphates are good ionic conductors,. Are Ionic Solids Good Conductors.
From www.nagwa.com
Question Video Explaining the Difference between Metals and Ionic Are Ionic Solids Good Conductors They are called fast ion. Ionic conductivity greatly varies between compounds. They have high melting and boiling points due to the strong ionic bonds. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds have high melting. Are Ionic Solids Good Conductors.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.9.1 Explaining Conductivity翰林国际教育 Are Ionic Solids Good Conductors Ionic conductivity greatly varies between compounds. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. They are called fast ion. They have high melting and boiling points due to the strong ionic bonds. A variety of crystalline phosphates are good ionic conductors,. Are Ionic Solids Good Conductors.
From slidetodoc.com
Ionic Conductivity and Solid Electrolytes I The Basics Are Ionic Solids Good Conductors Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. They have high melting and boiling points due to the strong ionic bonds. Ionic solids generally have high melting points and high boiling points. Ionic compounds are hard and brittle. They are called fast ion. This is a result of the strong. Are Ionic Solids Good Conductors.
From favpng.com
Ionic Compound Sodium Chloride Electrical Conductivity Water, PNG Are Ionic Solids Good Conductors They are called fast ion. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). Ionic compounds have high melting points. This is a result of the strong electrostatic. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds are hard. Are Ionic Solids Good Conductors.
From www.peoi.org
Chapter 10 Section D Solids Are Ionic Solids Good Conductors Ionic compounds are hard and brittle. Ionic conductivity greatly varies between compounds. They are called fast ion. This is a result of the strong electrostatic. Ionic solids generally have high melting points and high boiling points. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. Solutions of ionic compounds and melted. Are Ionic Solids Good Conductors.
From saylordotorg.github.io
Chemical Compounds Are Ionic Solids Good Conductors For some of them is much higher then for others. This is a result of the strong electrostatic. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. They are called fast ion. They have high melting and boiling points due to the. Are Ionic Solids Good Conductors.
From docslib.org
Chemical and Physical Properties of Sodium Ionic Conductors for Solid Are Ionic Solids Good Conductors Ionic solids generally have high melting points and high boiling points. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). Solutions of ionic compounds and melted ionic. Are Ionic Solids Good Conductors.
From www.youtube.com
Ionic solids Intermolecular forces and properties AP Chemistry Are Ionic Solids Good Conductors Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive. Are Ionic Solids Good Conductors.
From www.researchgate.net
Binaryion conductor and singleion conductor. Schematic illustration Are Ionic Solids Good Conductors They are called fast ion. Ionic compounds are hard and brittle. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). For some of them is much higher then for others. This is a result of the strong electrostatic. Ionic compounds do not conduct electricity as. Are Ionic Solids Good Conductors.
From www.youtube.com
Representing ionic solids using particulate models AP Chemistry Are Ionic Solids Good Conductors This is a result of the strong electrostatic. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na 1 + x zr 2 (po 4). Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds have high melting points. Ionic conductivity greatly varies between compounds. They are called. Are Ionic Solids Good Conductors.
From www.youtube.com
Ionic compounds, insulators and conductors YouTube Are Ionic Solids Good Conductors This is a result of the strong electrostatic. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds have high melting points. They have high melting and boiling points due to the strong ionic bonds. They are called fast ion. Ionic solids generally have high melting points and high boiling points. Ionic compounds do not. Are Ionic Solids Good Conductors.
From www.researchgate.net
Schematic representation showing the mechanism of ionic conduction in Are Ionic Solids Good Conductors Ionic conductivity greatly varies between compounds. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. This is a result of the strong electrostatic. Ionic compounds have high melting points. Ionic solids generally have high melting points and high boiling points. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being. Are Ionic Solids Good Conductors.
From www.w3schools.blog
Ionic Solids W3schools Are Ionic Solids Good Conductors This is a result of the strong electrostatic. Ionic compounds are hard and brittle. They have high melting and boiling points due to the strong ionic bonds. For some of them is much higher then for others. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. They are called fast ion.. Are Ionic Solids Good Conductors.
From studylib.net
Ionic Solids Characteristics Are Ionic Solids Good Conductors Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. For some of them is much higher then for others. Ionic compounds are hard and brittle. Ionic compounds have high melting points. Ionic conductivity greatly varies between compounds. A variety of crystalline phosphates. Are Ionic Solids Good Conductors.
From www.revision.my
5.7.1 Properties of Ionic and Covalent Compounds Revision.my Are Ionic Solids Good Conductors For some of them is much higher then for others. Ionic conductivity greatly varies between compounds. They are called fast ion. Ionic compounds have high melting points. This is a result of the strong electrostatic. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. A variety of crystalline phosphates are good. Are Ionic Solids Good Conductors.
From www.numerade.com
SOLVED Why are ionic solids poor electrical conductors in the solid Are Ionic Solids Good Conductors Ionic solids generally have high melting points and high boiling points. This is a result of the strong electrostatic. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. They are called fast ion. For some of them is much higher then for others. Ionic conductivity greatly varies between compounds. They have. Are Ionic Solids Good Conductors.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Are Ionic Solids Good Conductors They have high melting and boiling points due to the strong ionic bonds. Ionic conductivity greatly varies between compounds. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. Ionic compounds are hard and brittle. They are called fast ion. This is a result of the strong electrostatic. Ionic compounds do not. Are Ionic Solids Good Conductors.
From conceptoabc.com
Enlace iónico Qué es, concepto y definición Are Ionic Solids Good Conductors This is a result of the strong electrostatic. Ionic conductivity greatly varies between compounds. They have high melting and boiling points due to the strong ionic bonds. Ionic solids generally have high melting points and high boiling points. They are called fast ion. A variety of crystalline phosphates are good ionic conductors, notably nasicon (na super ionic conductor)—solid solutions na. Are Ionic Solids Good Conductors.
From byjus.com
Difference between ionic solid and compound solids Are Ionic Solids Good Conductors Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic conductivity greatly varies between compounds. Ionic compounds are hard and brittle. They have high melting and boiling points due to the strong ionic bonds. Solutions of ionic compounds and melted ionic compounds. Are Ionic Solids Good Conductors.
From www.researchgate.net
Classical examples of solid ionic conductors and their structures. (a Are Ionic Solids Good Conductors Ionic compounds have high melting points. For some of them is much higher then for others. This is a result of the strong electrostatic. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. They are called fast ion. Ionic conductivity greatly varies between compounds. Ionic compounds do not conduct electricity in. Are Ionic Solids Good Conductors.