Catalyst Formula . Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis is the process of speeding up a. Catalysts lower the activation energy barrier for a reaction without. A catalyst is a substrate that speeds up a reaction without being consumed. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Learn about different types of catalysts in chemistry and their role in chemical reactions. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many.
from www.numerade.com
A catalyst is a substrate that speeds up a reaction without being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts lower the activation energy barrier for a reaction without. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysis is the process of speeding up a. Enzymes are naturally occurring catalysts responsible for many. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition.
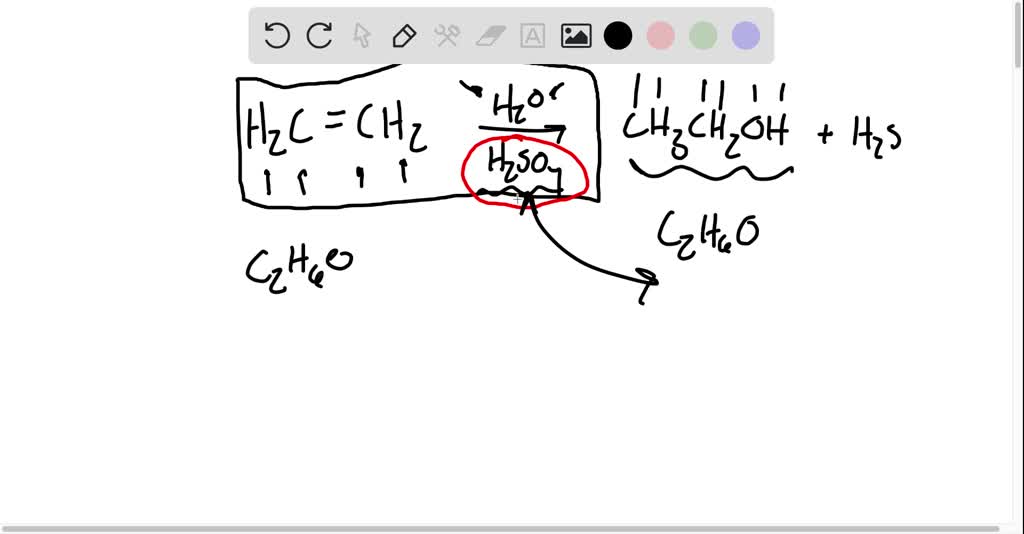
SOLVEDIdentify the catalyst in each equation.
Catalyst Formula A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts lower the activation energy barrier for a reaction without. A catalyst is a substrate that speeds up a reaction without being consumed. Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process of speeding up a. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Learn about different types of catalysts in chemistry and their role in chemical reactions. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalyst Formula Catalysis is the process of speeding up a. Catalysts lower the activation energy barrier for a reaction without. Enzymes are naturally occurring catalysts responsible for many. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst,. Catalyst Formula.
From www.youtube.com
01.09 General Acid Catalysis YouTube Catalyst Formula Learn about different types of catalysts in chemistry and their role in chemical reactions. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysis is the process of speeding up a. Catalysts have no effect on the equilibrium constant and thus on the equilibrium. Catalyst Formula.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalyst Formula A catalyst is a substrate that speeds up a reaction without being consumed. Enzymes are naturally occurring catalysts responsible for many. Catalysts lower the activation energy barrier for a reaction without. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts allow a reaction to proceed via. Catalyst Formula.
From ar.inspiredpencil.com
Catalyst Equation Catalyst Formula Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts lower the activation energy barrier for a reaction without. Catalysis is the process of speeding up a. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy. Catalyst Formula.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Formula Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up. Catalyst Formula.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalyst Formula Catalysis is the process of speeding up a. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn about different types of catalysts in chemistry and their role in chemical reactions. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it.. Catalyst Formula.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Catalyst Formula Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process of speeding up a. Enzymes are naturally occurring catalysts responsible for many. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition.. Catalyst Formula.
From www.slideserve.com
PPT Alkynes PowerPoint Presentation, free download ID221726 Catalyst Formula Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalyst, in. Catalyst Formula.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Formula Catalysts lower the activation energy barrier for a reaction without. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Learn about different types of catalysts in chemistry and their role in chemical reactions. Enzymes are naturally occurring catalysts responsible for many. In chemistry and biology, a catalyst is a substance the increases the rate of. Catalyst Formula.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID3206932 Catalyst Formula Catalysts lower the activation energy barrier for a reaction without. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst is a substrate that speeds up a reaction without being consumed. In chemistry and biology, a catalyst is a substance the increases the. Catalyst Formula.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Formula Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts have no effect on. Catalyst Formula.
From ar.inspiredpencil.com
Catalyst Equation Catalyst Formula Learn about different types of catalysts in chemistry and their role in chemical reactions. A catalyst is a substrate that speeds up a reaction without being consumed. Catalysis is the process of speeding up a. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. In chemistry and biology, a catalyst is a substance the increases. Catalyst Formula.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalyst Formula A catalyst is a substrate that speeds up a reaction without being consumed. Learn about different types of catalysts in chemistry and their role in chemical reactions. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process of speeding up a. In. Catalyst Formula.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Formula A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical. Catalyst Formula.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Formula A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being. Catalyst Formula.
From www.chemistrystudent.com
Catalysts (ALevel) ChemistryStudent Catalyst Formula Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts lower the activation energy barrier for. Catalyst Formula.
From www.slideserve.com
PPT Chemistry 102(01) Spring 2012 PowerPoint Presentation, free Catalyst Formula In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysis is the process of speeding up a. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that. Catalyst Formula.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Catalyst Formula Learn about different types of catalysts in chemistry and their role in chemical reactions. A catalyst is a substrate that speeds up a reaction without being consumed. Catalysts lower the activation energy barrier for a reaction without. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. Catalyst Formula.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalyst Formula Catalysis is the process of speeding up a. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts lower the activation energy barrier for a reaction without. Learn about different types of catalysts. Catalyst Formula.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Formula Catalysis is the process of speeding up a. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts lower the activation energy barrier for a reaction without. Catalysts have no effect on the. Catalyst Formula.
From www.slideserve.com
PPT Heterogeneous Catalysis & Solid State Physics PowerPoint Catalyst Formula Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis is the process of speeding up a. Enzymes are naturally occurring catalysts responsible for many. Catalysts have no effect on the equilibrium constant and thus on. Catalyst Formula.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalyst Formula Learn about different types of catalysts in chemistry and their role in chemical reactions. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Enzymes are naturally occurring catalysts responsible for many. Catalysts allow. Catalyst Formula.
From study.com
Wilkinson's Catalyst Formula, Structure & Applications Lesson Catalyst Formula Catalysts lower the activation energy barrier for a reaction without. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysis is the process of speeding up a. A catalyst is a substrate that speeds up a reaction without being. Catalyst Formula.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Formula Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts lower the activation energy barrier for a reaction without. A catalyst is a substrate that. Catalyst Formula.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Catalyst Formula Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts lower the activation energy barrier for a reaction without. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. In chemistry and biology, a catalyst. Catalyst Formula.
From www.essentialchemicalindustry.org
Catalysis in industry Catalyst Formula A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst is a substrate that speeds up a reaction without being consumed. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself. Catalyst Formula.
From www.numerade.com
SOLVED Question 7 formula written on the arrow in a chemical equation Catalyst Formula Catalysis is the process of speeding up a. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Enzymes are naturally occurring catalysts responsible for many. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Learn about different types of. Catalyst Formula.
From www.numerade.com
SOLVED write a chemical reaction which is carried out by catalyst with Catalyst Formula A catalyst is a substrate that speeds up a reaction without being consumed. Learn about different types of catalysts in chemistry and their role in chemical reactions. Enzymes are naturally occurring catalysts responsible for many. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysis is the process of speeding up a. Catalysts allow a. Catalyst Formula.
From www.numerade.com
SOLVEDIdentify the catalyst in each equation. Catalyst Formula A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysis is the process of speeding up a. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. Catalysts allow a. Catalyst Formula.
From slideplayer.com
Chemical Reactions. ppt download Catalyst Formula Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts lower the activation energy barrier for a reaction without. Learn about different types of catalysts in chemistry and their role. Catalyst Formula.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalyst Formula A catalyst is a substrate that speeds up a reaction without being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts lower the activation energy barrier for a reaction without. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that increases the rate of. Catalyst Formula.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Formula Catalysts lower the activation energy barrier for a reaction without. A catalyst is a substrate that speeds up a reaction without being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst. Catalyst Formula.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalyst Formula Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. A catalyst is a substrate that speeds up a reaction without being consumed. Catalysts lower the activation energy barrier for a reaction without. Catalyst,. Catalyst Formula.
From www.researchgate.net
Scheme 3. Chemical equation for the catalytic cleavage of the βO4 Catalyst Formula In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts lower the activation energy barrier for a reaction without. Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process of speeding up a. Catalysts have no effect on the equilibrium constant and thus on the. Catalyst Formula.
From www.numerade.com
SOLVED hydrocarbon of unknown structure has the formula CgHio On Catalyst Formula Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process of speeding up a. Catalysts lower the activation energy barrier for a reaction without. A catalyst is a substrate that speeds up a reaction without being consumed. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalyst, in chemistry, any substance that increases. Catalyst Formula.