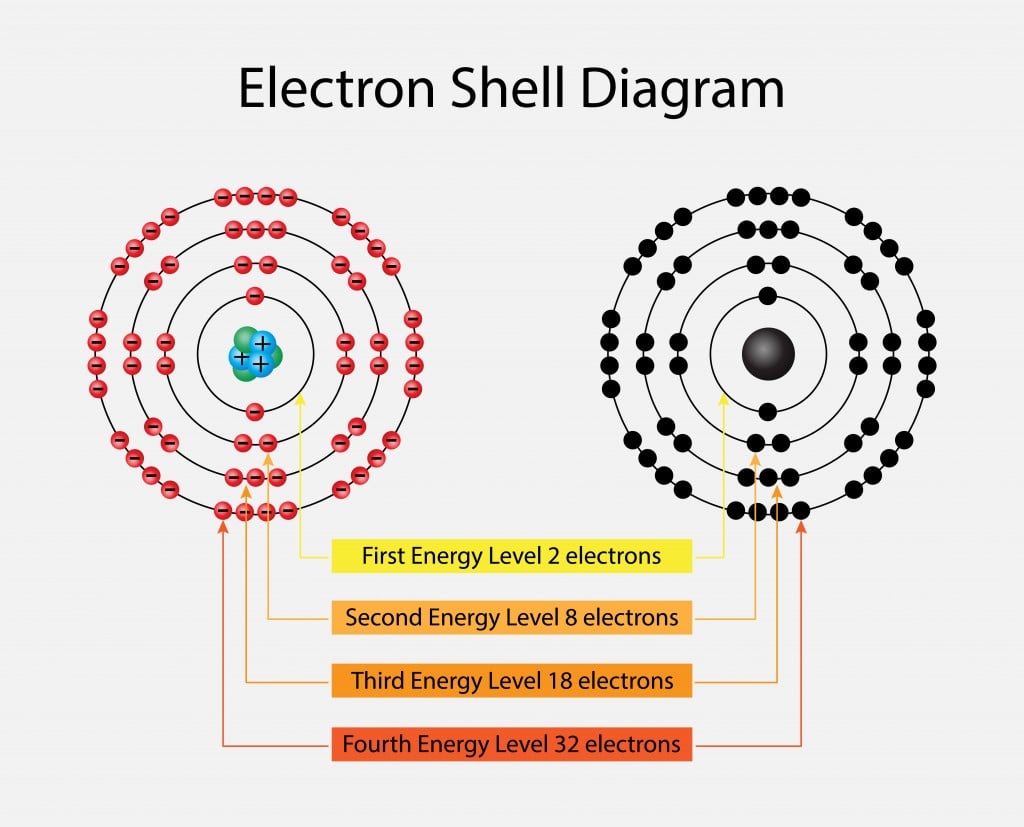
What Are Electron Levels . Energy levels are regions around the nucleus in which electrons move according to their energies. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electron energy levels are numbered, with higher levels having more energy. According to bohr's theory, electrons of. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Electrons are in the lowest energy level possible, unless excited by. Atoms have the lowest energy levels closest to. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. After filling the first shell level (with just an s subshell),. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. An atom is thought to contain several.
from www.scienceabc.com
Energy levels are regions around the nucleus in which electrons move according to their energies. Electron energy levels are numbered, with higher levels having more energy. According to bohr's theory, electrons of. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. After filling the first shell level (with just an s subshell),. An atom is thought to contain several. Atoms have the lowest energy levels closest to. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Electrons are in the lowest energy level possible, unless excited by.
Octet Rule Why Are Atoms With 8 Valence Electrons So Stable?
What Are Electron Levels In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. After filling the first shell level (with just an s subshell),. Atoms have the lowest energy levels closest to. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. Electrons are in the lowest energy level possible, unless excited by. Electron energy levels are numbered, with higher levels having more energy. Energy levels are regions around the nucleus in which electrons move according to their energies. According to bohr's theory, electrons of. An atom is thought to contain several. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found.
From sciencenotes.org
List of Electron Configurations of Elements What Are Electron Levels An atom is thought to contain several. Energy levels are regions around the nucleus in which electrons move according to their energies. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. Electrons are in the lowest energy level possible, unless excited by. According to bohr's theory, electrons of. Atoms. What Are Electron Levels.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table What Are Electron Levels The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. According to bohr's theory, electrons of. Electrons are in the lowest energy level possible, unless excited by. An atom is thought to. What Are Electron Levels.
From www.slideserve.com
PPT Electron Configurations PowerPoint Presentation, free download What Are Electron Levels In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. After filling the first shell level (with just an s subshell),. According to bohr's theory, electrons of. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom.. What Are Electron Levels.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Are Electron Levels After filling the first shell level (with just an s subshell),. Energy levels are regions around the nucleus in which electrons move according to their energies. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. In this section we will discuss the energy level of the electron of a. What Are Electron Levels.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy What Are Electron Levels Electrons are in the lowest energy level possible, unless excited by. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Atoms have the lowest energy levels. What Are Electron Levels.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Are Electron Levels After filling the first shell level (with just an s subshell),. Energy levels are regions around the nucleus in which electrons move according to their energies. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Atoms have the lowest energy levels closest to. Electron energy levels are numbered, with. What Are Electron Levels.
From cronodon.com
Introduction to Atoms What Are Electron Levels According to bohr's theory, electrons of. Electrons are in the lowest energy level possible, unless excited by. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. An atom is thought to. What Are Electron Levels.
From www.slideserve.com
PPT Electron Orbitals PowerPoint Presentation, free download ID650435 What Are Electron Levels An atom is thought to contain several. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. After filling the first shell level (with just. What Are Electron Levels.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube What Are Electron Levels In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Electrons are in the lowest energy level possible, unless excited by. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Atoms have the lowest. What Are Electron Levels.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png What Are Electron Levels The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Electron energy levels are numbered, with higher levels having more energy. Electrons are in the lowest energy level possible, unless excited by. After filling the first shell level (with just an s subshell),. Energy levels (also called electron shells) are fixed distances. What Are Electron Levels.
From quizlet.com
Diagram and label electron energy levels in Bohr's model. Quizlet What Are Electron Levels Electrons are in the lowest energy level possible, unless excited by. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. After filling the first shell level. What Are Electron Levels.
From www.slideserve.com
PPT Introduction to Chemistry PowerPoint Presentation, free download What Are Electron Levels In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Energy levels are regions around the nucleus in which electrons move according to their energies. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found.. What Are Electron Levels.
From mavink.com
Electron Energy Levels Chart What Are Electron Levels Electron energy levels are numbered, with higher levels having more energy. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Atoms have the lowest energy levels closest to. The arrangement of electrons in the orbitals of an atom is called the electron configuration of. What Are Electron Levels.
From gzscienceclassonline.weebly.com
1. Electron Configuration What Are Electron Levels According to bohr's theory, electrons of. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. An atom is thought to contain several. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electron energy levels are numbered, with higher levels having. What Are Electron Levels.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica What Are Electron Levels In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the. What Are Electron Levels.
From boisestate.pressbooks.pub
3.4 Electronic Structure of Atoms (Electron Configurations) General What Are Electron Levels An atom is thought to contain several. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Energy levels are regions around the nucleus in. What Are Electron Levels.
From www.thoughtco.com
Electron Configuration Chart What Are Electron Levels According to bohr's theory, electrons of. An atom is thought to contain several. Electrons are in the lowest energy level possible, unless excited by. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. In this section we will discuss the energy level of the electron of a hydrogen atom, and how. What Are Electron Levels.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation, free download ID5479879 What Are Electron Levels An atom is thought to contain several. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. Electrons are in the lowest energy level possible, unless excited by. Atoms have the lowest energy levels closest to. Energy levels are regions around the nucleus in which electrons move according to their. What Are Electron Levels.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron What Are Electron Levels After filling the first shell level (with just an s subshell),. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. An atom is thought to contain several. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes. What Are Electron Levels.
From learningcampusdustin.z4.web.core.windows.net
Electron Energy Levels Chart What Are Electron Levels Electron energy levels are numbered, with higher levels having more energy. Electrons are in the lowest energy level possible, unless excited by. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Atoms have the lowest energy levels closest to. Energy levels are regions around. What Are Electron Levels.
From stock.adobe.com
chart of electron configuration with each energy level for element in What Are Electron Levels In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. After filling the first shell level (with just an s subshell),. Atoms have the lowest energy levels closest to. The arrangement of electrons in the orbitals of an atom is called the electron configuration of. What Are Electron Levels.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes What Are Electron Levels After filling the first shell level (with just an s subshell),. Energy levels are regions around the nucleus in which electrons move according to their energies. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electron energy levels are numbered, with higher levels having more energy. The arrangement of. What Are Electron Levels.
From www.physics.udel.edu
Electron Energy Levels What Are Electron Levels Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electron energy levels are numbered, with higher levels having more energy. Electrons are in the lowest energy level possible, unless excited by. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the. What Are Electron Levels.
From www.periodictableprintable.com
Periodic Table With Electron Structure Of Energy Levels 2023 Periodic What Are Electron Levels After filling the first shell level (with just an s subshell),. Energy levels are regions around the nucleus in which electrons move according to their energies. According to bohr's theory, electrons of. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Electron energy levels. What Are Electron Levels.
From scientifictutor.org
Chem Complete Electron Configurations Scientific Tutor What Are Electron Levels Electrons are in the lowest energy level possible, unless excited by. Electron energy levels are numbered, with higher levels having more energy. Atoms have the lowest energy levels closest to. After filling the first shell level (with just an s subshell),. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Energy. What Are Electron Levels.
From www.scienceabc.com
Octet Rule Why Are Atoms With 8 Valence Electrons So Stable? What Are Electron Levels Energy levels are regions around the nucleus in which electrons move according to their energies. Electrons are in the lowest energy level possible, unless excited by. An atom is thought to contain several. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Electron energy levels are numbered, with higher levels having. What Are Electron Levels.
From www.stonecoldhands.com
Electron Configurations First Three Rows Stone Cold Chemistry Talk What Are Electron Levels Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. An atom is thought to contain several. After filling the first shell level (with just an s subshell),. Electrons are in the lowest energy level possible, unless excited by. In this section we will discuss the energy level of the. What Are Electron Levels.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and What Are Electron Levels In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. After filling the first shell level (with just an s subshell),. Atoms have the lowest energy levels closest to. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as. What Are Electron Levels.
From hubpages.com
Atoms and Atomic Structure HubPages What Are Electron Levels Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes. What Are Electron Levels.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts What Are Electron Levels In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. The arrangement of electrons in the orbitals of an atom is called the electron configuration. What Are Electron Levels.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica What Are Electron Levels Electrons are in the lowest energy level possible, unless excited by. Electron energy levels are numbered, with higher levels having more energy. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. According to bohr's theory, electrons of. Atoms have the lowest energy levels closest to. After filling the first. What Are Electron Levels.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Carlson Stock Art What Are Electron Levels In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Atoms have the lowest energy levels closest to. An atom is thought to contain several. According to bohr's theory, electrons of. Electrons are in the lowest energy level possible, unless excited by. Energy levels (also. What Are Electron Levels.
From www.ck12.org
The Periodic Table and Electron Configurations CK12 Foundation What Are Electron Levels In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. An atom is thought to contain several. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. According to bohr's theory, electrons of. Energy levels (also called. What Are Electron Levels.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table What Are Electron Levels Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. According to bohr's theory, electrons of. Energy levels are regions around the nucleus in which electrons move according to their. What Are Electron Levels.
From www.slideserve.com
PPT Modern Atomic Theory PowerPoint Presentation, free download ID What Are Electron Levels An atom is thought to contain several. Energy levels are regions around the nucleus in which electrons move according to their energies. In a classical bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. What Are Electron Levels.