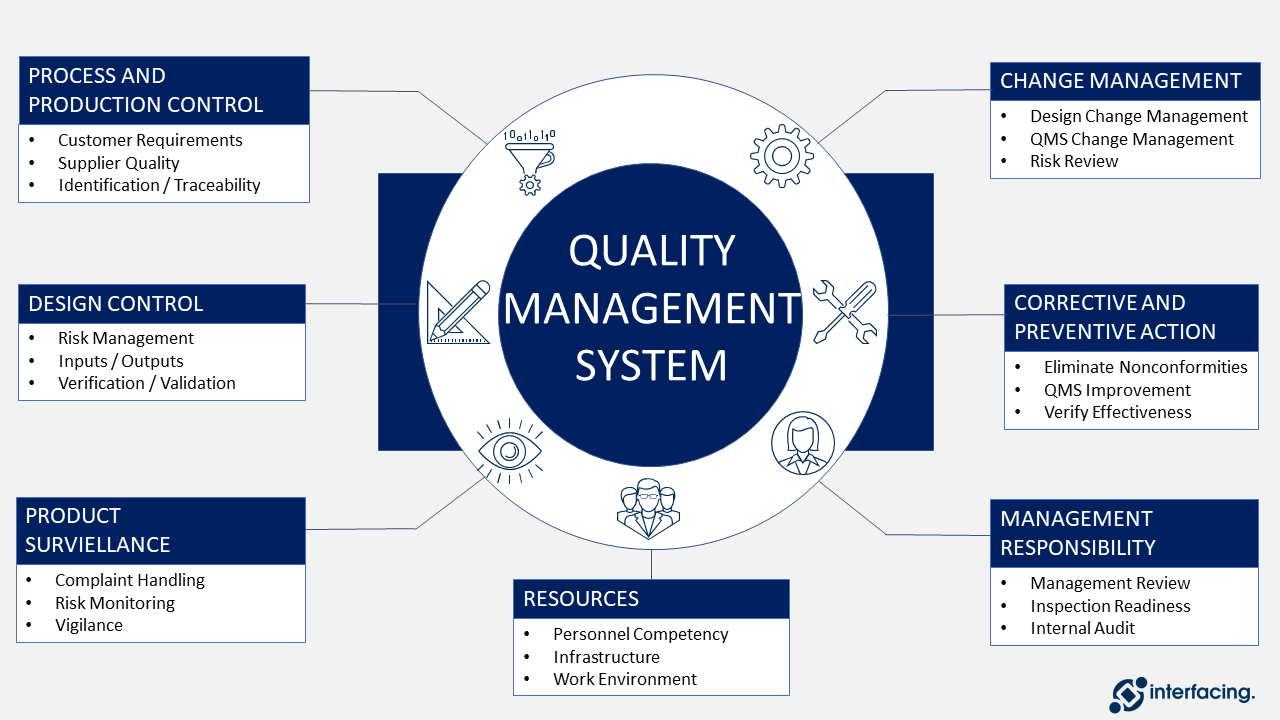
Medical Device Quality Management System Fda . On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. 10k+ visitors in the past month On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. On february 2, 2024, the u.s. The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to. With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. 10k+ visitors in the past month
from www.interfacing.com
With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. 10k+ visitors in the past month 10k+ visitors in the past month The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. On february 2, 2024, the u.s. The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to. On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice.
Medtech FDA QSR, ISO 13845, EUDAMED, conformité MEDTECH
Medical Device Quality Management System Fda 10k+ visitors in the past month The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to. The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. 10k+ visitors in the past month The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. 10k+ visitors in the past month On february 2, 2024, the u.s. On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice.
From www.slideserve.com
PPT Introduction to Quality Management Systems for Medical Devices Medical Device Quality Management System Fda 10k+ visitors in the past month Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good.. Medical Device Quality Management System Fda.
From medicaldevicehq.com
A guide to quality management for medical devices and ISO 13485 Medical Device Quality Management System Fda The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. On february 2, 2024, the u.s. The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory. Medical Device Quality Management System Fda.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Device Quality Management System Fda 10k+ visitors in the past month 10k+ visitors in the past month On february 2, 2024, the u.s. Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. With the february 2, 2026. Medical Device Quality Management System Fda.
From www.joharidigital.com
Importance of Quality Management System in Medical Device Manufacturing Medical Device Quality Management System Fda Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. The medical device single audit program (mdsap). Medical Device Quality Management System Fda.
From www.aplyon.com
Medical Device Quality Management System MANUFACTURING Medical Device Quality Management System Fda The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to. The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. The fda issued the quality management system regulation (qmsr) final rule, which amends. Medical Device Quality Management System Fda.
From medicaldevicehq.com
A guide to quality management for medical devices and ISO 13485 Medical Device Quality Management System Fda Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. 10k+ visitors in the past month On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good.. Medical Device Quality Management System Fda.
From www.greenlight.guru
Ultimate Guide to ISO 13485 Quality Management System (QMS) for Medical Medical Device Quality Management System Fda On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. On february 2, 2024, the u.s. With the february 2, 2026 compliance deadline approaching, medical device. Medical Device Quality Management System Fda.
From www.cognidox.com
4 ways to build a medical device quality management system Medical Device Quality Management System Fda The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to. On february 2, 2024, the u.s. On january 31, 2024, the fda issued. Medical Device Quality Management System Fda.
From www.scribd.com
FDA Software Development PDF Medical Device Quality Management System Medical Device Quality Management System Fda Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. On january 21, 2024, the fda. Medical Device Quality Management System Fda.
From blog.greenlight.guru
ISO 13485 and FDA QSR A StepbyStep Guide to Complying with Medical Medical Device Quality Management System Fda On february 2, 2024, the u.s. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. 10k+ visitors in the past month With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. The medical device single audit program (mdsap) is a program that allows the conduct of. Medical Device Quality Management System Fda.
From intellisoft.io
Medical Device QMS What Is It And How To Build a QMS Medical Device Quality Management System Fda 10k+ visitors in the past month The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. 10k+ visitors in the past month On january 21, 2024, the fda issued a final rule amending its. Medical Device Quality Management System Fda.
From quality.eqms.co.uk
ISO 134852016 6 tips to optimise your medical device quality Medical Device Quality Management System Fda 10k+ visitors in the past month With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. On february 2, 2024, the u.s. Food and drug administration (“fda”) published a final rule 1 to amend the current good. Medical Device Quality Management System Fda.
From www.aplyon.com
Medical Device Quality Management System MANUFACTURING Medical Device Quality Management System Fda On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to. 10k+ visitors in the past month On january 21, 2024, the fda. Medical Device Quality Management System Fda.
From www.greenlight.guru
QMSR (Quality Management System Regulation) Explained What FDA QSR Medical Device Quality Management System Fda 10k+ visitors in the past month The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part. Medical Device Quality Management System Fda.
From www.slideserve.com
PPT Introduction to Quality Management Systems for Medical Devices Medical Device Quality Management System Fda On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. The medical device single audit program (mdsap) is a program that allows the conduct of a. Medical Device Quality Management System Fda.
From www.complianceonline.com
ISO 134852016 Requirement Medical Devices Quality Management Systems Medical Device Quality Management System Fda 10k+ visitors in the past month On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to. On february. Medical Device Quality Management System Fda.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Device Quality Management System Fda 10k+ visitors in the past month 10k+ visitors in the past month On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. The new regulation, referred to as the quality management system regulation,. Medical Device Quality Management System Fda.
From www.aplyon.com
Medical Device Quality Management System DESIGN PLUS Medical Device Quality Management System Fda The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality. Medical Device Quality Management System Fda.
From enhancequality.com
FDA ISO 13485 Elevate Medical Device Quality Management Medical Device Quality Management System Fda On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device.. Medical Device Quality Management System Fda.
From thewitfire.in
Understanding ISO 13485 A Guide to Medical Device Quality Management Medical Device Quality Management System Fda On february 2, 2024, the u.s. 10k+ visitors in the past month 10k+ visitors in the past month On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. The new regulation, referred to. Medical Device Quality Management System Fda.
From worldcomplianceseminars.com
Medical Device Quality Management System Course WCS Medical Device Quality Management System Fda On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. On february 2, 2024, the u.s. The new regulation, referred to as the quality management system regulation, or qmsr. Medical Device Quality Management System Fda.
From iziel.com
QMS Documentation for Medical Devices ISO 13485 Certification IZiel Medical Device Quality Management System Fda On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. On february 2, 2024, the u.s. 10k+ visitors in the past month The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. With the february 2, 2026 compliance deadline approaching, medical device manufacturers. Medical Device Quality Management System Fda.
From advancedsafety.com.sg
ISO 13485 Quality Management System for Medical Devices o Singapore Medical Device Quality Management System Fda 10k+ visitors in the past month On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. Food and drug administration (“fda”) published a final rule 1. Medical Device Quality Management System Fda.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Device Quality Management System Fda The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. 10k+ visitors in the past month On. Medical Device Quality Management System Fda.
From www.youtube.com
European Medical Device Registration Chapter 3 Quality Management Medical Device Quality Management System Fda The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good.. Medical Device Quality Management System Fda.
From insights.tuv.com
Publication of ISO134852016 Quality System Standard Medical Device Quality Management System Fda The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to. 10k+ visitors in the past month With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. The fda issued the quality management system regulation (qmsr) final. Medical Device Quality Management System Fda.
From gaca-usa.com
ISO13485 Medical Devices Quality Management System Medical Device Quality Management System Fda The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. On february 2, 2024, the u.s. With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. 10k+ visitors in the past month 10k+ visitors in the past month On january 21, 2024, the fda issued a final. Medical Device Quality Management System Fda.
From www.slideserve.com
PPT FDA Medical Device Quality System Introduction PowerPoint Medical Device Quality Management System Fda On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. 10k+ visitors in the past month On. Medical Device Quality Management System Fda.
From poddtoppen.se
QMSR The Future of FDA's Quality Management System Regulation for Medical Device Quality Management System Fda On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp) requirements of. The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. With the february 2,. Medical Device Quality Management System Fda.
From www.interfacing.com
Medtech FDA QSR, ISO 13845, EUDAMED, conformité MEDTECH Medical Device Quality Management System Fda 10k+ visitors in the past month On february 2, 2024, the u.s. 10k+ visitors in the past month On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. On january 31, 2024, the fda issued a final rule amending the device current good manufacturing practice (cgmp). Medical Device Quality Management System Fda.
From allevents.in
US FDA Medical Device QSR, 21 CFR 820 and Quality Management System Medical Device Quality Management System Fda On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. On february 2, 2024, the u.s. The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. Food and drug administration (“fda”) published a final rule 1 to. Medical Device Quality Management System Fda.
From www.iso-saudi.net
ISO 13485 Certification using USFDA approved ISO Saudi Arabia Medical Device Quality Management System Fda On january 21, 2024, the fda issued a final rule amending its quality system (qs) regulations under 21 cfr part 820, which addresses current. The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to. 10k+ visitors in the past month On february. Medical Device Quality Management System Fda.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key Medical Device Quality Management System Fda 10k+ visitors in the past month On february 2, 2024, the u.s. Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. The new regulation, referred to as the quality management system regulation, or qmsr for short, is the medical device. On january 31, 2024, the fda issued a final rule amending the. Medical Device Quality Management System Fda.
From www.joharidigital.com
Importance of Quality Management System in Medical Device Manufacturing Medical Device Quality Management System Fda 10k+ visitors in the past month With the february 2, 2026 compliance deadline approaching, medical device manufacturers must prepare for fda’s updated. 10k+ visitors in the past month The fda issued the quality management system regulation (qmsr) final rule, which amends the device current good. The new regulation, referred to as the quality management system regulation, or qmsr for short,. Medical Device Quality Management System Fda.
From www.freyrsolutions.com
Quality Management System (QMS) in Medical Devices Freyr Global Medical Device Quality Management System Fda 10k+ visitors in the past month The medical device single audit program (mdsap) is a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system to. On february 2, 2024, the u.s. Food and drug administration (“fda”) published a final rule 1 to amend the current good manufacturing practice. With the february. Medical Device Quality Management System Fda.