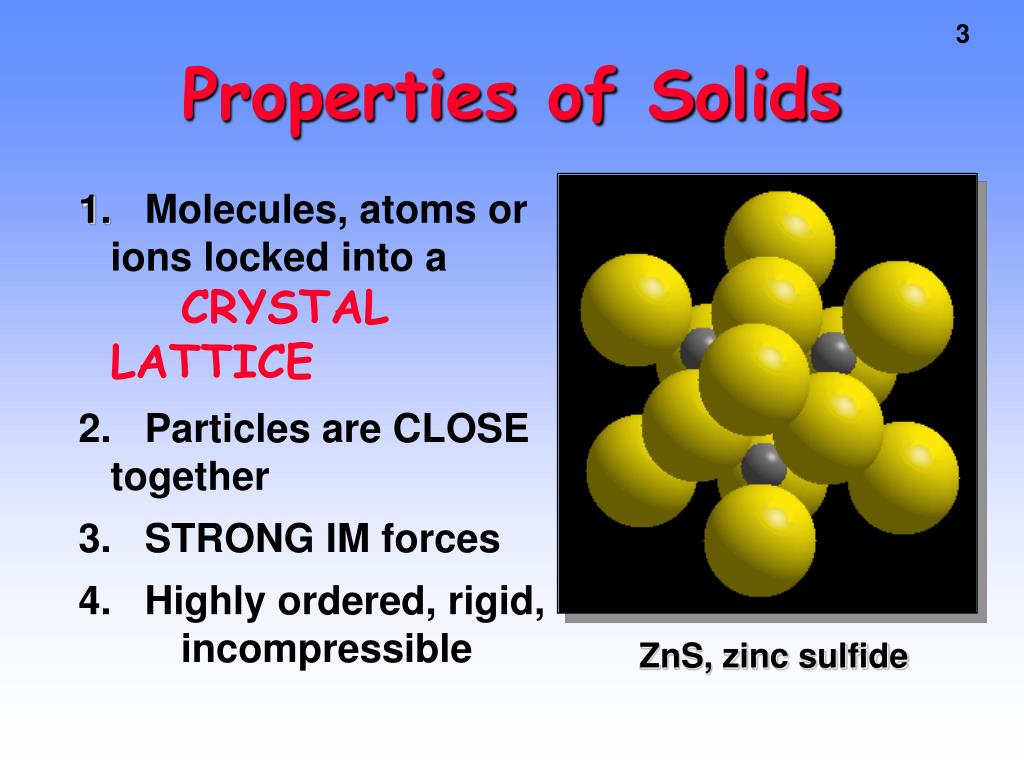
Properties Of Solid In Points . Solid are characterized by structural rigidity and resistance to changes of shape or volume. By the end of this section, you will be able to: To understand the correlation between bonding and the properties of solids. In fact, these forces are. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Define and describe the bonding and properties of ionic, molecular,. They have fixed shape size and volume. Solid is one of the three main states of matter, along with liquid and gas. Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. They are incompressible and inflexible. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. The particles constituting a solid may be atoms (e.g, in metal),. Unlike a liquid, a solid object does not.
from www.slideserve.com
Unlike a liquid, a solid object does not. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. Define and describe the bonding and properties of ionic, molecular,. To understand the correlation between bonding and the properties of solids. By the end of this section, you will be able to: They are incompressible and inflexible. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. In fact, these forces are. They have fixed shape size and volume.
PPT Types of Solids PowerPoint Presentation, free download ID4500701
Properties Of Solid In Points They have fixed shape size and volume. Solid are characterized by structural rigidity and resistance to changes of shape or volume. Define and describe the bonding and properties of ionic, molecular,. Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. In fact, these forces are. Solid is one of the three main states of matter, along with liquid and gas. By the end of this section, you will be able to: Unlike a liquid, a solid object does not. The particles constituting a solid may be atoms (e.g, in metal),. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. They have fixed shape size and volume. To understand the correlation between bonding and the properties of solids. They are incompressible and inflexible.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID Properties Of Solid In Points Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. In fact, these forces are. Solid are characterized by structural rigidity and resistance to changes of shape or volume. To understand the correlation between bonding and the properties of solids. Solid is one of. Properties Of Solid In Points.
From www.slideserve.com
PPT Types of Solid PowerPoint Presentation, free download ID2628261 Properties Of Solid In Points The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. By the end of this section, you will be able to: In fact, these forces are. Solid is one of the three main states of matter, along with liquid and gas. Matter is the stuff of the universe, the atoms, molecules and ions that make. Properties Of Solid In Points.
From www.askiitians.com
Classification of Solids Study Material for IIT JEE askIITians Properties Of Solid In Points Unlike a liquid, a solid object does not. They have fixed shape size and volume. Define and describe the bonding and properties of ionic, molecular,. To understand the correlation between bonding and the properties of solids. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Solid are characterized by structural rigidity and resistance to. Properties Of Solid In Points.
From www.slideserve.com
PPT Solids PowerPoint Presentation, free download ID1993152 Properties Of Solid In Points Unlike a liquid, a solid object does not. To understand the correlation between bonding and the properties of solids. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. They have fixed shape size and volume. The. Properties Of Solid In Points.
From studylib.net
physical properties of solids (student handout Properties Of Solid In Points The particles constituting a solid may be atoms (e.g, in metal),. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. They have fixed shape size and volume. They are incompressible and inflexible. Unlike a liquid, a solid object does not. Define and describe the bonding and properties of ionic, molecular,. Solids are generally held. Properties Of Solid In Points.
From www.slideserve.com
PPT Structure of Matter PowerPoint Presentation, free download ID Properties Of Solid In Points The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Solid are characterized by structural rigidity and resistance to changes of shape or volume. Solid is one of the three main states of matter, along with liquid and gas. Define and describe the bonding and properties of ionic, molecular,. Matter is the stuff of the. Properties Of Solid In Points.
From sciencenotes.org
What Is a Solid? Definition and Examples in Science Properties Of Solid In Points In fact, these forces are. Solid is one of the three main states of matter, along with liquid and gas. Solid are characterized by structural rigidity and resistance to changes of shape or volume. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong.. Properties Of Solid In Points.
From guides.byjusweb.com
What are Solid Shapes? Properties, Nets, Plane Figure, Videos, Examples Properties Of Solid In Points They are incompressible and inflexible. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. By the end of this section, you will be able to: Unlike a liquid, a solid object does not. They have fixed shape size and volume. Solid is one. Properties Of Solid In Points.
From studylib.net
Structure and Properties of SOLIDS Properties Of Solid In Points They are incompressible and inflexible. By the end of this section, you will be able to: They have fixed shape size and volume. Define and describe the bonding and properties of ionic, molecular,. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. The. Properties Of Solid In Points.
From www.youtube.com
Mechanical properties of solids Best Handwritten Notes Class 11 Properties Of Solid In Points By the end of this section, you will be able to: Solid are characterized by structural rigidity and resistance to changes of shape or volume. In fact, these forces are. Unlike a liquid, a solid object does not. They are incompressible and inflexible. They have fixed shape size and volume. Solids are generally held together by ionic or strong covalent. Properties Of Solid In Points.
From www.slideserve.com
PPT Properties of Solids PowerPoint Presentation, free download ID Properties Of Solid In Points By the end of this section, you will be able to: Solid is one of the three main states of matter, along with liquid and gas. They have fixed shape size and volume. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Matter is the stuff of the universe, the atoms, molecules and ions. Properties Of Solid In Points.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID4500701 Properties Of Solid In Points The particles constituting a solid may be atoms (e.g, in metal),. Solid is one of the three main states of matter, along with liquid and gas. Define and describe the bonding and properties of ionic, molecular,. In fact, these forces are. To understand the correlation between bonding and the properties of solids. They have fixed shape size and volume. The. Properties Of Solid In Points.
From www.ck12.org
Comparing Crystals Overview ( Video ) Chemistry CK12 Foundation Properties Of Solid In Points The particles constituting a solid may be atoms (e.g, in metal),. Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. Define and describe the bonding and properties of ionic, molecular,. To understand the correlation between bonding and the properties of solids. They have fixed shape size and volume. Solids are generally. Properties Of Solid In Points.
From www.slideserve.com
PPT PROPERTIES OF MATTER PowerPoint Presentation, free download ID Properties Of Solid In Points They are incompressible and inflexible. By the end of this section, you will be able to: Solid is one of the three main states of matter, along with liquid and gas. Unlike a liquid, a solid object does not. Define and describe the bonding and properties of ionic, molecular,. They have fixed shape size and volume. The properties of solids. Properties Of Solid In Points.
From www.britannica.com
solid Definition & Facts Britannica Properties Of Solid In Points Solid is one of the three main states of matter, along with liquid and gas. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Matter is the stuff. Properties Of Solid In Points.
From www.slideserve.com
PPT PROPERTIES OF MATTER PowerPoint Presentation, free download ID Properties Of Solid In Points The particles constituting a solid may be atoms (e.g, in metal),. In fact, these forces are. By the end of this section, you will be able to: Solid are characterized by structural rigidity and resistance to changes of shape or volume. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Matter is the stuff. Properties Of Solid In Points.
From www.slideserve.com
PPT Physical Properties of Matter PowerPoint Presentation, free Properties Of Solid In Points The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. Unlike a liquid, a solid object does not. Solid are characterized by structural rigidity and resistance to changes of shape or volume. Define and describe the bonding. Properties Of Solid In Points.
From mungfali.com
Solid Liquid Gas Anchor Chart Properties Of Solid In Points Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. Unlike a liquid, a solid object does not. By the end of this section, you will be able to: Solid are characterized by structural rigidity and resistance to changes of shape or volume. Define and describe the bonding and properties of ionic,. Properties Of Solid In Points.
From www.slideserve.com
PPT Lecture 2 The Crystal Structure of Solids PowerPoint Presentation Properties Of Solid In Points To understand the correlation between bonding and the properties of solids. They are incompressible and inflexible. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Solid is one of the three main states of matter, along with liquid and gas. Matter is the stuff of the universe, the atoms, molecules and ions that make. Properties Of Solid In Points.
From pt.slideshare.net
Solid shapes Properties Of Solid In Points They have fixed shape size and volume. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. By the end of this section, you will be able to: They are incompressible and inflexible. The properties of solids include definite shape and volume, strong intermolecular. Properties Of Solid In Points.
From www.pinterest.com
Geometric Solids Education elementary math, Math geometry, Basic math Properties Of Solid In Points By the end of this section, you will be able to: Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. The particles constituting a solid. Properties Of Solid In Points.
From www.studocu.com
The first section in chapter 12 The properties of solids 12. Melting Properties Of Solid In Points They have fixed shape size and volume. Unlike a liquid, a solid object does not. The particles constituting a solid may be atoms (e.g, in metal),. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. Define and describe the bonding and properties of. Properties Of Solid In Points.
From www.slideserve.com
PPT Properties of Solids PowerPoint Presentation, free download ID Properties Of Solid In Points They have fixed shape size and volume. They are incompressible and inflexible. Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. Define and describe the bonding and properties of ionic, molecular,. Solid are characterized by structural rigidity and resistance to changes of shape or volume. To understand the correlation between bonding. Properties Of Solid In Points.
From dokumen.tips
(PDF) Structure and Properties of Solids DOKUMEN.TIPS Properties Of Solid In Points Define and describe the bonding and properties of ionic, molecular,. Solid are characterized by structural rigidity and resistance to changes of shape or volume. Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. They are incompressible and inflexible. Solid is one of the three main states of matter, along with liquid. Properties Of Solid In Points.
From www.breakingatom.com
States of Matter Properties Of Solid In Points Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. They are incompressible and inflexible. Define and describe the bonding and properties of ionic, molecular,. In fact, these forces are. Solid are characterized by structural rigidity and resistance to changes of shape or volume. By the end of this section, you will. Properties Of Solid In Points.
From www.slideserve.com
PPT SOLID STATES PowerPoint Presentation, free download ID6018703 Properties Of Solid In Points To understand the correlation between bonding and the properties of solids. They are incompressible and inflexible. Solid are characterized by structural rigidity and resistance to changes of shape or volume. In fact, these forces are. Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very. Properties Of Solid In Points.
From www.etutorworld.com
What is a Solid? Properties Of Solid In Points The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. In fact, these forces are. Solid are characterized by structural rigidity and resistance to changes of shape or volume. By the end of this section, you will be able to: The particles constituting a solid may be atoms (e.g, in metal),. Solid is one of. Properties Of Solid In Points.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID4500685 Properties Of Solid In Points Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. By the end of this section, you will be able to: Solid are characterized by structural rigidity and resistance to changes of shape or volume. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Define and. Properties Of Solid In Points.
From www.slideserve.com
PPT Properties of Matter PowerPoint Presentation, free download ID Properties Of Solid In Points Solid is one of the three main states of matter, along with liquid and gas. In fact, these forces are. Define and describe the bonding and properties of ionic, molecular,. By the end of this section, you will be able to: Solid are characterized by structural rigidity and resistance to changes of shape or volume. Solids are generally held together. Properties Of Solid In Points.
From www.slideshare.net
States of matter ppt Properties Of Solid In Points They are incompressible and inflexible. Solid is one of the three main states of matter, along with liquid and gas. Solid are characterized by structural rigidity and resistance to changes of shape or volume. By the end of this section, you will be able to: They have fixed shape size and volume. The properties of solids include definite shape and. Properties Of Solid In Points.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Properties Of Solid In Points Solid are characterized by structural rigidity and resistance to changes of shape or volume. In fact, these forces are. They have fixed shape size and volume. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. By the end of this section, you will be able to: Unlike a liquid, a solid object does not.. Properties Of Solid In Points.
From www.slideserve.com
PPT Properties of Solids PowerPoint Presentation, free download ID Properties Of Solid In Points Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. The particles constituting a solid may be atoms (e.g, in metal),. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. They have fixed shape size and volume. Solid is one of the three main states of. Properties Of Solid In Points.
From www.teachercreated.com
Solid Geometric Shapes Chart TCR7779 Teacher Created Resources Properties Of Solid In Points Define and describe the bonding and properties of ionic, molecular,. To understand the correlation between bonding and the properties of solids. They have fixed shape size and volume. By the end of this section, you will be able to: In fact, these forces are. The properties of solids include definite shape and volume, strong intermolecular forces, and low compressibility. Solid. Properties Of Solid In Points.
From eduinput.com
Crystalline solids Properties, types, examples use Properties Of Solid In Points Solids are generally held together by ionic or strong covalent bonding, and the attractive forces between the atoms, ions, or molecules in solids are very strong. Solid is one of the three main states of matter, along with liquid and gas. Unlike a liquid, a solid object does not. Matter is the stuff of the universe, the atoms, molecules and. Properties Of Solid In Points.
From www.cuemath.com
Visualizing Solid Shapes Faces, Vertices, Edges of solid shapes, How Properties Of Solid In Points The particles constituting a solid may be atoms (e.g, in metal),. In fact, these forces are. Matter is the stuff of the universe, the atoms, molecules and ions that make up all physical substances. Solid is one of the three main states of matter, along with liquid and gas. The properties of solids include definite shape and volume, strong intermolecular. Properties Of Solid In Points.