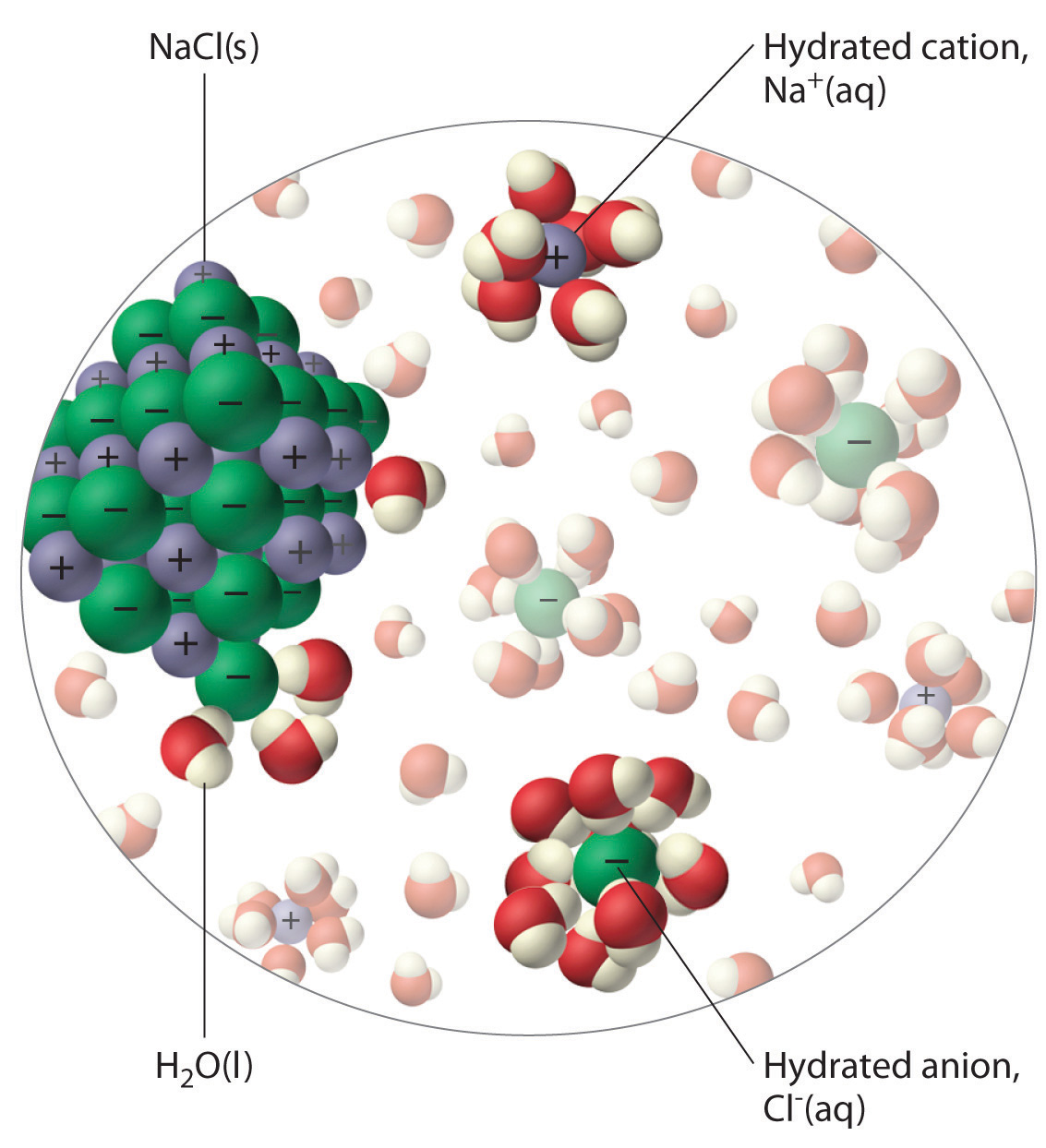
Solid Zinc Nitrate Dissolves In Water . water typically dissolves most ionic compounds and polar molecules. beads of oil in water. Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: It reacts with bases to make zinc hydroxide. in this video we will describe the equation zn (no3)2 + h2o and write what. Zinc nitrate is a colorless solid. When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate:
from chem.libretexts.org
for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Zinc nitrate is a colorless solid. water typically dissolves most ionic compounds and polar molecules. in this video we will describe the equation zn (no3)2 + h2o and write what. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. Nonpolar molecules, such as those found in grease. When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: It reacts with bases to make zinc hydroxide.
4.1 General Properties of Aqueous Solutions Chemistry LibreTexts
Solid Zinc Nitrate Dissolves In Water for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: water typically dissolves most ionic compounds and polar molecules. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Zinc nitrate is a colorless solid. beads of oil in water. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. It reacts with bases to make zinc hydroxide. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Nonpolar molecules, such as those found in grease. in this video we will describe the equation zn (no3)2 + h2o and write what. When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads.
From giojvhjpj.blob.core.windows.net
Does Zinc React With Hydrogen Peroxide at Guadalupe Jenning blog Solid Zinc Nitrate Dissolves In Water It reacts with bases to make zinc hydroxide. beads of oil in water. water typically dissolves most ionic compounds and polar molecules. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for. Solid Zinc Nitrate Dissolves In Water.
From www.numerade.com
SOLVED 'Question 4 Compounds in Aqueous Solution The compound ammonium Solid Zinc Nitrate Dissolves In Water Zinc nitrate is a colorless solid. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: water typically dissolves most ionic. Solid Zinc Nitrate Dissolves In Water.
From www.youtube.com
The action of heat on zinc II nitrate Laboratory experiments on salts Solid Zinc Nitrate Dissolves In Water Nonpolar molecules, such as those found in grease. water typically dissolves most ionic compounds and polar molecules. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. beads of oil in water. for example, solid zinc nitrate. Solid Zinc Nitrate Dissolves In Water.
From 2012books.lardbucket.org
Describing Electrochemical Cells Solid Zinc Nitrate Dissolves In Water beads of oil in water. When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. Nonpolar molecules, such as those found in grease. in this video we will describe the equation zn (no3)2 + h2o and write what. It reacts with bases to make. Solid Zinc Nitrate Dissolves In Water.
From www.sciencephoto.com
Zinc nitrate crystals Stock Image A710/0062 Science Photo Library Solid Zinc Nitrate Dissolves In Water for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: in this video we will describe the equation zn (no3)2 + h2o and write what. It reacts with bases to make zinc hydroxide. When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not. Solid Zinc Nitrate Dissolves In Water.
From slideplayer.com
Chapter 13 Aqueous Solutions ppt download Solid Zinc Nitrate Dissolves In Water \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. Zinc nitrate is a colorless solid. Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: beads of oil in water.. Solid Zinc Nitrate Dissolves In Water.
From www.numerade.com
SOLVED The compound sodlum nitrate strong electrolyte. Write the Solid Zinc Nitrate Dissolves In Water Zinc nitrate is a colorless solid. beads of oil in water. in this video we will describe the equation zn (no3)2 + h2o and write what. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. water typically dissolves most ionic compounds and polar. Solid Zinc Nitrate Dissolves In Water.
From www.youtube.com
Equation for Zn(NO3)2 + H2O (Zinc nitrate + Water) YouTube Solid Zinc Nitrate Dissolves In Water for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: It reacts with bases to make zinc hydroxide. water typically dissolves most ionic compounds and polar molecules. When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. . Solid Zinc Nitrate Dissolves In Water.
From www.researchgate.net
Morphologies of ZnO particles formed from zinc nitrate synthesized as Solid Zinc Nitrate Dissolves In Water Zinc nitrate is a colorless solid. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. It reacts with bases to make zinc hydroxide. beads of oil in water. in this video we will describe the equation zn (no3)2 + h2o and write what. Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate dissolves in water to. Solid Zinc Nitrate Dissolves In Water.
From chem.libretexts.org
4.1 General Properties of Aqueous Solutions Chemistry LibreTexts Solid Zinc Nitrate Dissolves In Water for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Zinc nitrate is a colorless solid. beads of oil in water. It reacts with bases to make zinc hydroxide. Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate dissolves in water to form an aqueous solution of. Solid Zinc Nitrate Dissolves In Water.
From www.researchgate.net
Color Change of Zinc Nitrate Solution by Yarrowia lipolytica Solid Zinc Nitrate Dissolves In Water for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: in this video we will describe the equation zn (no3)2 + h2o and write what. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: beads of oil in water. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow. Solid Zinc Nitrate Dissolves In Water.
From www.youtube.com
What is the product of Zinc nitrate (Zn (NO3)2) & Sodium hydroxide Solid Zinc Nitrate Dissolves In Water in this video we will describe the equation zn (no3)2 + h2o and write what. It reacts with bases to make zinc hydroxide. Nonpolar molecules, such as those found in grease. water typically dissolves most ionic compounds and polar molecules. Zinc nitrate is a colorless solid. beads of oil in water. for example, solid zinc nitrate. Solid Zinc Nitrate Dissolves In Water.
From collegedunia.com
Zinc Nitrate Structure, Formula, Preparation, Properties & Uses Solid Zinc Nitrate Dissolves In Water beads of oil in water. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate. Solid Zinc Nitrate Dissolves In Water.
From www.youtube.com
What is the reaction between Zinc nitrate (Zn(NO3)2) and sodium Solid Zinc Nitrate Dissolves In Water beads of oil in water. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Zinc nitrate is a colorless solid. for example, solid zinc nitrate dissolves in. Solid Zinc Nitrate Dissolves In Water.
From www.researchgate.net
SEM image of the aspreparedZnOsample prepared using zinc nitrate Solid Zinc Nitrate Dissolves In Water Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. water typically dissolves most ionic compounds and polar molecules. . Solid Zinc Nitrate Dissolves In Water.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Solid Zinc Nitrate Dissolves In Water Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Zinc nitrate is a colorless solid. When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. for example, solid zinc. Solid Zinc Nitrate Dissolves In Water.
From www.chegg.com
Solved Which of the following is the correct dissociation Solid Zinc Nitrate Dissolves In Water Zinc nitrate is a colorless solid. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: water typically dissolves most ionic compounds and polar molecules. beads of oil. Solid Zinc Nitrate Dissolves In Water.
From fphoto.photoshelter.com
science chemistry exothermic reaction iodine zinc Fundamental Solid Zinc Nitrate Dissolves In Water beads of oil in water. water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Zinc nitrate is a colorless solid. It reacts with bases to make zinc hydroxide. for example, solid zinc. Solid Zinc Nitrate Dissolves In Water.
From www.youtube.com
The Thermodynamics of Potassium Nitrate Dissolving in Water YouTube Solid Zinc Nitrate Dissolves In Water in this video we will describe the equation zn (no3)2 + h2o and write what. Zinc nitrate is a colorless solid. water typically dissolves most ionic compounds and polar molecules. beads of oil in water. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc. Solid Zinc Nitrate Dissolves In Water.
From www.youtube.com
Zinc metal reaction Nitric acid reaction Zinc in nitric acid Solid Zinc Nitrate Dissolves In Water Zinc nitrate is a colorless solid. beads of oil in water. in this video we will describe the equation zn (no3)2 + h2o and write what. Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves. Solid Zinc Nitrate Dissolves In Water.
From www.slideserve.com
PPT Na 3 PO 4 + 3 KOH â 3 NaOH + K 3 PO 4 Double Displacement Solid Zinc Nitrate Dissolves In Water Nonpolar molecules, such as those found in grease. beads of oil in water. Zinc nitrate is a colorless solid. It reacts with bases to make zinc hydroxide. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. in. Solid Zinc Nitrate Dissolves In Water.
From www.showme.com
Zinc nitrate Science, Chemistry, Chemical Bonds ShowMe Solid Zinc Nitrate Dissolves In Water in this video we will describe the equation zn (no3)2 + h2o and write what. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: It reacts with bases to make zinc hydroxide. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Zinc nitrate. Solid Zinc Nitrate Dissolves In Water.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Solid Zinc Nitrate Dissolves In Water When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. Nonpolar molecules, such as those found in grease. in this video we will describe the equation zn (no3)2 + h2o and write what. beads of oil in water. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +.. Solid Zinc Nitrate Dissolves In Water.
From flatworldknowledge.lardbucket.org
Reactions in Aqueous Solution Solid Zinc Nitrate Dissolves In Water When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. in this video we will describe the equation zn (no3)2 + h2o and write what. Zinc nitrate is a colorless solid. for example, solid zinc nitrate dissolves in water to form an aqueous solution. Solid Zinc Nitrate Dissolves In Water.
From 2012books.lardbucket.org
The Dissolution Process Solid Zinc Nitrate Dissolves In Water water typically dissolves most ionic compounds and polar molecules. in this video we will describe the equation zn (no3)2 + h2o and write what. beads of oil in water. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: When a nonpolar liquid such as oil is dispersed in a. Solid Zinc Nitrate Dissolves In Water.
From www.youtube.com
Zinc Powder in water YouTube Solid Zinc Nitrate Dissolves In Water for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Zinc nitrate is a colorless solid. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: water typically. Solid Zinc Nitrate Dissolves In Water.
From www.researchgate.net
Crystal stacking of ZnO (a) and zinc nitrate hydroxide (b); schematic Solid Zinc Nitrate Dissolves In Water water typically dissolves most ionic compounds and polar molecules. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: When a nonpolar liquid such as oil is dispersed in a polar solvent such as water,. Solid Zinc Nitrate Dissolves In Water.
From fphoto.photoshelter.com
science chemistry precipitation reaction silver chloride water Solid Zinc Nitrate Dissolves In Water \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: water typically dissolves most ionic compounds and polar molecules. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: beads of oil in water. Zinc nitrate is a. Solid Zinc Nitrate Dissolves In Water.
From www.numerade.com
SOLVED The compound zinc bromide, ZnBr2 is soluble in water. Write the Solid Zinc Nitrate Dissolves In Water Zinc nitrate is a colorless solid. beads of oil in water. water typically dissolves most ionic compounds and polar molecules. in this video we will describe the equation zn (no3)2 + h2o and write what. Nonpolar molecules, such as those found in grease. for example, solid zinc nitrate dissolves in water to form an aqueous solution. Solid Zinc Nitrate Dissolves In Water.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Solid Zinc Nitrate Dissolves In Water When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: It reacts with bases to make zinc hydroxide. Nonpolar molecules, such as those found in grease. Zinc nitrate is. Solid Zinc Nitrate Dissolves In Water.
From www.slideserve.com
PPT Chapter 8 “Chemical Reactions” PowerPoint Presentation, free Solid Zinc Nitrate Dissolves In Water When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. Nonpolar molecules, such as those found in grease. water typically dissolves most ionic compounds and polar molecules. It reacts with bases to make zinc hydroxide. Zinc nitrate is a colorless solid. in this video. Solid Zinc Nitrate Dissolves In Water.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Solid Zinc Nitrate Dissolves In Water \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. beads of oil in water. When a nonpolar liquid such as oil is dispersed in a polar solvent such as water, it does not dissolve, but forms spherical beads. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: water typically dissolves most ionic compounds. Solid Zinc Nitrate Dissolves In Water.
From www.shutterstock.com
Zinc Nitrate Molecule Illustration Vector Stock Vector (Royalty Free Solid Zinc Nitrate Dissolves In Water It reacts with bases to make zinc hydroxide. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: Zinc nitrate is a colorless solid. \[\ce{zn(no3)2(s) + h2o(l) \rightarrow zn^{2+}(aq) +. Nonpolar molecules, such as those found. Solid Zinc Nitrate Dissolves In Water.
From www.researchgate.net
Simplified configuration of zinc reactions with water Download Solid Zinc Nitrate Dissolves In Water for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: beads of oil in water. water typically dissolves most ionic compounds and polar molecules. Zinc nitrate is a colorless solid. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: When a nonpolar liquid. Solid Zinc Nitrate Dissolves In Water.
From giocupxbz.blob.core.windows.net
Zinc Aqueous Color at Lillian Imai blog Solid Zinc Nitrate Dissolves In Water Zinc nitrate is a colorless solid. It reacts with bases to make zinc hydroxide. for example, solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate: in this video we will describe the equation zn (no3)2 + h2o and write what. for example, solid zinc nitrate dissolves in water to form an aqueous. Solid Zinc Nitrate Dissolves In Water.