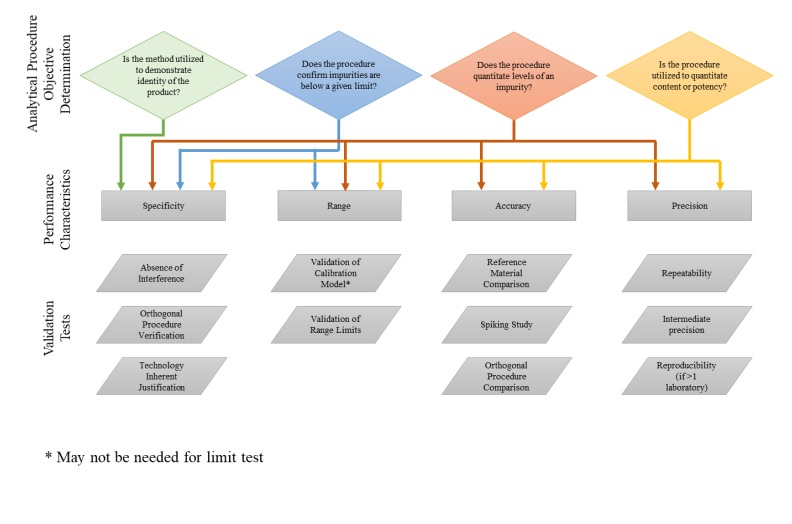
Calibration And Validation As Per Ich . each of these validation characteristics is defined in the attached glossary. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. The table lists those validation characteristics. In the second phase, model validation, a validation set with independent samples is used for validation of the.
from pharmasciences.in
The table lists those validation characteristics. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. each of these validation characteristics is defined in the attached glossary. the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). In the second phase, model validation, a validation set with independent samples is used for validation of the. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an.
ICH Q2 (R1) PharmaSciences
Calibration And Validation As Per Ich together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. In the second phase, model validation, a validation set with independent samples is used for validation of the. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. The table lists those validation characteristics. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). each of these validation characteristics is defined in the attached glossary.
From www.pharmaceuticalsky.com
Differences between Calibration, Verification and Validation Calibration And Validation As Per Ich each of these validation characteristics is defined in the attached glossary. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. In the second phase, model validation, a validation set with independent samples is used for validation of the. in november 2005, the international council. Calibration And Validation As Per Ich.
From www.youtube.com
Basic Definitions of Calibration, verification and validation Calibration And Validation As Per Ich it provides guidance and recommendations on how to derive and evaluate the various validation tests for. In the second phase, model validation, a validation set with independent samples is used for validation of the. each of these validation characteristics is defined in the attached glossary. at step 2 of the ich process, a consensus draft text or. Calibration And Validation As Per Ich.
From pharmabeej.com
ICH Guideline Q2 Analytical Validation Pharmabeej Calibration And Validation As Per Ich it provides guidance and recommendations on how to derive and evaluate the various validation tests for. In the second phase, model validation, a validation set with independent samples is used for validation of the. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. The table. Calibration And Validation As Per Ich.
From www.slideshare.net
DATION OF EQUIPMENT ICH AND WHO GUIDELINES FOR CALIBRATION AND VALIDA… Calibration And Validation As Per Ich In the second phase, model validation, a validation set with independent samples is used for validation of the. The table lists those validation characteristics. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich. Calibration And Validation As Per Ich.
From www.slideshare.net
DATION OF EQUIPMENT ICH AND WHO GUIDELINES FOR CALIBRATION AND VALIDA… Calibration And Validation As Per Ich at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. The. Calibration And Validation As Per Ich.
From www.pharmacytimess.com
Difference among Calibration, Validation and Qualification Calibration And Validation As Per Ich in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. In the second phase, model validation, a validation set with independent samples is used for validation of the. each of these validation. Calibration And Validation As Per Ich.
From www.researchgate.net
Schematic representation of the calibration and validation process for Calibration And Validation As Per Ich The table lists those validation characteristics. In the second phase, model validation, a validation set with independent samples is used for validation of the. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. each of these validation characteristics is defined in the attached glossary. . Calibration And Validation As Per Ich.
From www.researchgate.net
Flowchart of calibration and validation Download Scientific Diagram Calibration And Validation As Per Ich at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of. Calibration And Validation As Per Ich.
From www.youtube.com
Difference between Calibration, Qualification and Validation YouTube Calibration And Validation As Per Ich In the second phase, model validation, a validation set with independent samples is used for validation of the. each of these validation characteristics is defined in the attached glossary. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). together ich q14 and ich q2(r2) describe the development and validation. Calibration And Validation As Per Ich.
From www.youtube.com
Calibration and Validation. Different between calibration and Calibration And Validation As Per Ich it provides guidance and recommendations on how to derive and evaluate the various validation tests for. each of these validation characteristics is defined in the attached glossary. In the second phase, model validation, a validation set with independent samples is used for validation of the. in november 2005, the international council for harmonisation of technical requirements for. Calibration And Validation As Per Ich.
From www.youtube.com
Calibration Vs Validation Differences explained with example Calibration And Validation As Per Ich in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. each of these validation characteristics is defined in the attached glossary. The table lists those validation characteristics. together ich q14 and. Calibration And Validation As Per Ich.
From xiltrixusa.com
Calibration and Validation in the Lab Calibration And Validation As Per Ich it provides guidance and recommendations on how to derive and evaluate the various validation tests for. In the second phase, model validation, a validation set with independent samples is used for validation of the. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. The table lists those validation characteristics.. Calibration And Validation As Per Ich.
From www.scribd.com
Computer System Validation ICH PDF Verification And Validation Calibration And Validation As Per Ich In the second phase, model validation, a validation set with independent samples is used for validation of the. The table lists those validation characteristics. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic. Calibration And Validation As Per Ich.
From www.slideshare.net
Analytical method validation as per ich and usp Calibration And Validation As Per Ich The table lists those validation characteristics. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). In the second phase, model validation, a validation set with independent samples is used for validation of the. each of these validation characteristics is defined in the attached glossary. the scope of the revision. Calibration And Validation As Per Ich.
From www.youtube.com
Calibration Vs Validation What is the Difference ? [2023 Calibration And Validation As Per Ich together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. each of these validation characteristics is defined in the attached glossary. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. the scope of the revision. Calibration And Validation As Per Ich.
From pharmainterview.com
What is the difference between calibration, verification, validation Calibration And Validation As Per Ich in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. each of these validation characteristics is defined in the attached glossary. at step 2 of the ich process, a consensus draft. Calibration And Validation As Per Ich.
From www.slideshare.net
Ich & who guidelines for calibration and validation 22 PPT Calibration And Validation As Per Ich together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. In the second phase, model validation, a validation set with independent samples is used for validation of the. it provides guidance. Calibration And Validation As Per Ich.
From www.slideshare.net
Analytical method validation as per ich and usp Calibration And Validation As Per Ich at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. . Calibration And Validation As Per Ich.
From calibrationawareness.com
How to Differentiate Calibration, Verification, and Validation Calibration And Validation As Per Ich in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). In the second phase, model validation, a validation set with independent samples is used for validation of the. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. . Calibration And Validation As Per Ich.
From www.slideshare.net
DATION OF EQUIPMENT ICH AND WHO GUIDELINES FOR CALIBRATION AND VALIDA… Calibration And Validation As Per Ich at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. . Calibration And Validation As Per Ich.
From pharmaguddu.com
Difference Between Validation, Calibration, and Qualification in Pharma Calibration And Validation As Per Ich it provides guidance and recommendations on how to derive and evaluate the various validation tests for. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). In the second phase, model validation, a validation set with independent samples is used for validation of the. at step 2 of the ich. Calibration And Validation As Per Ich.
From www.pharmaspecialists.com
Difference Between Calibration and Validation Calibration And Validation As Per Ich at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. The table lists those validation characteristics. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. In the second phase, model validation, a validation set with independent samples. Calibration And Validation As Per Ich.
From www.researchgate.net
(PDF) ICH and WHO Guideline for validation and calibration Calibration And Validation As Per Ich together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. In the second phase, model validation, a validation set with independent samples is used for validation of the. the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. each of these. Calibration And Validation As Per Ich.
From mavink.com
Difference Between Calibration And Validation Calibration And Validation As Per Ich the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. The table lists those validation characteristics. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. at step 2 of the ich process, a consensus draft text or guideline, agreed by. Calibration And Validation As Per Ich.
From www.youtube.com
Differences Between Validation and Calibration YouTube Calibration And Validation As Per Ich the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. in november 2005, the. Calibration And Validation As Per Ich.
From pharmasciences.in
ICH Q2 (R1) PharmaSciences Calibration And Validation As Per Ich together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). each of these validation characteristics is defined in the attached glossary. the scope of the revision of ich q2(r1) includes validation. Calibration And Validation As Per Ich.
From www.youtube.com
Introduction to calibration and validation YouTube Calibration And Validation As Per Ich each of these validation characteristics is defined in the attached glossary. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. The table lists those validation characteristics. the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. it provides guidance. Calibration And Validation As Per Ich.
From www.slideserve.com
PPT GEOGG142 GMES Calibration & validation of EO products PowerPoint Calibration And Validation As Per Ich The table lists those validation characteristics. it provides guidance and recommendations on how to derive and evaluate the various validation tests for. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). In the second phase, model validation, a validation set with independent samples is used for validation of the. . Calibration And Validation As Per Ich.
From www.slideshare.net
Analytical method validation as per ich and usp Calibration And Validation As Per Ich The table lists those validation characteristics. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. each of these validation characteristics is defined in the attached glossary. . Calibration And Validation As Per Ich.
From www.youtube.com
Calibration and validation YouTube Calibration And Validation As Per Ich the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. In the second phase, model validation, a validation set with independent samples is used for validation of the. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). The table lists those validation. Calibration And Validation As Per Ich.
From www.westgard.com
Calibration Verification Criteria for Acceptable Performance Westgard Calibration And Validation As Per Ich at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). In the second phase, model validation, a validation set with independent samples is used for validation of the. . Calibration And Validation As Per Ich.
From analysisdoo.com
Calibration and validation Analysis Calibration And Validation As Per Ich the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. each of these validation characteristics is defined in the attached glossary. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. at step 2 of the ich process, a consensus. Calibration And Validation As Per Ich.
From pharmabeej.com
Q3 ICH Guidelines For Impurities Pharmabeej Calibration And Validation As Per Ich in november 2005, the international council for harmonisation of technical requirements for pharmaceuticals for human use (ich). In the second phase, model validation, a validation set with independent samples is used for validation of the. the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. The table lists those validation. Calibration And Validation As Per Ich.
From www.slideshare.net
Analytical method validation as per ich and usp Calibration And Validation As Per Ich In the second phase, model validation, a validation set with independent samples is used for validation of the. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. each of these validation characteristics is defined in the attached glossary. the scope of the revision of ich q2(r1) includes validation. Calibration And Validation As Per Ich.
From www.researchgate.net
Illustration of the calibration and validation process Download Calibration And Validation As Per Ich the scope of the revision of ich q2(r1) includes validation principles that cover analytical use of spectroscopic or. The table lists those validation characteristics. each of these validation characteristics is defined in the attached glossary. together ich q14 and ich q2(r2) describe the development and validation activities suggested during the lifecycle of an. at step 2. Calibration And Validation As Per Ich.