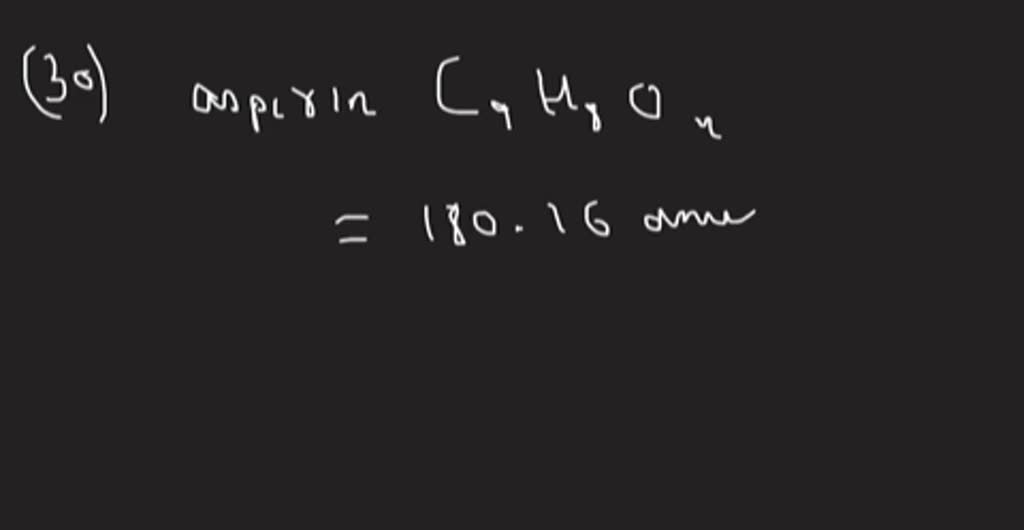Moles Aspirin Formula . knowing the mass of each element in a compound we can determine its formula. Convert grams aspirin to moles. now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. Molar mass of c9h8o4 = 180.15742 g/mol. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. molar mass of aspirin = 180.16 g. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. In order to dissolve aspirin completely in water, you. There are two types of formulas, empirical and molecular.
from www.numerade.com
Convert grams aspirin to moles. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. knowing the mass of each element in a compound we can determine its formula. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. In order to dissolve aspirin completely in water, you. There are two types of formulas, empirical and molecular. Molar mass of c9h8o4 = 180.15742 g/mol. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. the chemical name of the active ingredient in aspirin is acetylsalicylic acid.
SOLVEDHow many moles of aspirin, C9 H8 O4, are in a 500 mg tablet?
Moles Aspirin Formula Molar mass of c9h8o4 = 180.15742 g/mol. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. knowing the mass of each element in a compound we can determine its formula. Molar mass of c9h8o4 = 180.15742 g/mol. molar mass of aspirin = 180.16 g. There are two types of formulas, empirical and molecular. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. In order to dissolve aspirin completely in water, you. Convert grams aspirin to moles. now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance.
From www.coursehero.com
[Solved] Determine the number of moles of aspirin if a student weighed Moles Aspirin Formula the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16). Moles Aspirin Formula.
From www.dreamstime.com
Acetylsalicylic Acid or Aspirin Drug Molecule. Skeletal Formula Stock Moles Aspirin Formula Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. Convert grams aspirin to moles. now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. Molar mass of c9h8o4 = 180.15742 g/mol. knowing the mass of each. Moles Aspirin Formula.
From www.coursehero.com
[Solved] O a, 8.3x105 . How many moles of aspirin (CoH402, 180 g/mol Moles Aspirin Formula Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. There are two types of formulas, empirical and molecular. In order to dissolve aspirin completely in water, you. Molar mass of c9h8o4 = 180.15742 g/mol. Convert grams aspirin to. Moles Aspirin Formula.
From www.dreamstime.com
Acetylsalicylic Acid, Aspirin, Structural Chemical Formula Stock Photo Moles Aspirin Formula Convert grams aspirin to moles. There are two types of formulas, empirical and molecular. In order to dissolve aspirin completely in water, you. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. molar mass of aspirin = 180.16 g. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. now that you know. Moles Aspirin Formula.
From www.chegg.com
Solved How many moles of O are in 0.355 mole of aspirin, Moles Aspirin Formula Molar mass of c9h8o4 = 180.15742 g/mol. There are two types of formulas, empirical and molecular. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. molar mass of aspirin = 180.16 g. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. In order to dissolve aspirin. Moles Aspirin Formula.
From www.numerade.com
SOLVED Calculate the theoretical yield of aspirin (FW = 180.158 g/mol Moles Aspirin Formula molar mass of aspirin = 180.16 g. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. Molar mass of c9h8o4 = 180.15742 g/mol. There are two types of formulas, empirical and molecular. Convert grams aspirin to moles. knowing the mass of each element in a compound we can determine. Moles Aspirin Formula.
From www.youtube.com
theoretical yield of aspirin YouTube Moles Aspirin Formula now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. molar mass of aspirin = 180.16 g. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. The mechanism of action of aspirin is. Moles Aspirin Formula.
From www.coursehero.com
[Solved] If 0.25 moles of salicylic acid are used as the starting Moles Aspirin Formula the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. molar mass of aspirin = 180.16 g. There are two types of formulas, empirical and molecular. knowing the mass of each element in a compound we can determine its formula. Theoretical yield of aspirin (g) = (2. Moles Aspirin Formula.
From www.sliderbase.com
How many moles of hydrogen are in 100 L of hydrogen at STP? 100 L = mol Moles Aspirin Formula Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. knowing the mass of each element in a compound we can determine its formula. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. In order to dissolve aspirin completely. Moles Aspirin Formula.
From www.pinterest.com
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density Moles Aspirin Formula Molar mass of c9h8o4 = 180.15742 g/mol. There are two types of formulas, empirical and molecular. In order to dissolve aspirin completely in water, you. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. knowing the mass of each element in a compound we can determine its. Moles Aspirin Formula.
From stock.adobe.com
Aspirin Formula is given and explained here along with its structure Moles Aspirin Formula the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. Convert grams aspirin to moles. Molar mass of c9h8o4 = 180.15742 g/mol. now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. In order to. Moles Aspirin Formula.
From www.numerade.com
SOLVED Text Page 63 Question (1 point) An aspirin tablet contains Moles Aspirin Formula molar mass of aspirin = 180.16 g. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. In order to dissolve aspirin completely in water, you. Convert grams aspirin to moles. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3. Moles Aspirin Formula.
From www.numerade.com
SOLVED How many moles of aspirin are in one tablet? Moles Aspirin Formula The mechanism of action of aspirin is as a cyclooxygenase inhibitor. molar mass of aspirin = 180.16 g. In order to dissolve aspirin completely in water, you. Molar mass of c9h8o4 = 180.15742 g/mol. Convert grams aspirin to moles. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. There are. Moles Aspirin Formula.
From www.numerade.com
SOLVED How many moles of aspirin, C9H8O4, are in a bottle containing Moles Aspirin Formula the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. Molar mass of c9h8o4 = 180.15742 g/mol. Convert grams aspirin to moles. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. molar mass of aspirin = 180.16 g. In order to dissolve aspirin completely. Moles Aspirin Formula.
From solvedlib.com
Moles Question How many moles of aspirin; 8 are mo… SolvedLib Moles Aspirin Formula There are two types of formulas, empirical and molecular. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. Molar mass of c9h8o4 = 180.15742 g/mol. now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. In order. Moles Aspirin Formula.
From socratic.org
Question ce7a4 Socratic Moles Aspirin Formula now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Molar mass of c9h8o4 = 180.15742 g/mol. molar mass of aspirin = 180.16 g. the chemical name of the active ingredient in aspirin. Moles Aspirin Formula.
From www.numerade.com
SOLVED Aspirin can be prepared from salicylic acid (C7H6O3) which has Moles Aspirin Formula Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. molar mass of aspirin = 180.16 g. now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a. Moles Aspirin Formula.
From www.numerade.com
SOLVED During this lab, the following balanced reaction will be done Moles Aspirin Formula There are two types of formulas, empirical and molecular. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. In order to dissolve aspirin completely in water, you. the molecular mass of aspirin is (9 × 12) +. Moles Aspirin Formula.
From cartoondealer.com
Aspirin Molecular Structure Stock Image 5174365 Moles Aspirin Formula In order to dissolve aspirin completely in water, you. molar mass of aspirin = 180.16 g. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. There are two types of formulas, empirical and molecular. knowing the mass of each element in a compound we can determine. Moles Aspirin Formula.
From ar.inspiredpencil.com
Acetylsalicylic Acid Formula Moles Aspirin Formula the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. In order to dissolve aspirin completely in water, you. knowing the mass of each element in a compound we can determine its formula. The mechanism. Moles Aspirin Formula.
From www.numerade.com
SOLVEDHow many moles of aspirin, C9 H8 O4, are in a 500 mg tablet? Moles Aspirin Formula the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. Convert grams aspirin to moles. knowing the mass of each element in a compound we can determine its formula. Molar mass of c9h8o4 = 180.15742 g/mol. the chemical name of the active ingredient in aspirin is acetylsalicylic. Moles Aspirin Formula.
From www.numerade.com
SOLVED Problem7 i) Calculate the molar mass (molecular weight) of Moles Aspirin Formula knowing the mass of each element in a compound we can determine its formula. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. There are two types of formulas, empirical and molecular. molar mass of aspirin. Moles Aspirin Formula.
From www.numerade.com
SOLVEDAspirin can be represented by the adjacent ballandstick Moles Aspirin Formula The mechanism of action of aspirin is as a cyclooxygenase inhibitor. knowing the mass of each element in a compound we can determine its formula. In order to dissolve aspirin completely in water, you. Molar mass of c9h8o4 = 180.15742 g/mol. There are two types of formulas, empirical and molecular. molar mass of aspirin = 180.16 g. . Moles Aspirin Formula.
From www.freepik.com
Premium Vector Aspirin chemistry chemical formula structure vector Moles Aspirin Formula The mechanism of action of aspirin is as a cyclooxygenase inhibitor. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. Molar mass of c9h8o4 = 180.15742 g/mol. Convert grams aspirin to moles. knowing the mass of each. Moles Aspirin Formula.
From www.numerade.com
SOLVED The molecular formula of acetylsalicylic acid (aspirin), one of Moles Aspirin Formula Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. There are two types of formulas, empirical and molecular. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. the chemical name of the active ingredient in aspirin is acetylsalicylic. Moles Aspirin Formula.
From www.numerade.com
SOLVED 9 Question (1 point) An aspirin tablet contains 81.0 Moles Aspirin Formula In order to dissolve aspirin completely in water, you. knowing the mass of each element in a compound we can determine its formula. Molar mass of c9h8o4 = 180.15742 g/mol. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16. Moles Aspirin Formula.
From www.coursehero.com
[Solved] 12. Carefully move your filter paper with aspirin crystals to Moles Aspirin Formula the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. There are. Moles Aspirin Formula.
From www.chegg.com
Solved How many moles of aspirin, C9H304, are in a 5.00 x 10 Moles Aspirin Formula Molar mass of c9h8o4 = 180.15742 g/mol. There are two types of formulas, empirical and molecular. knowing the mass of each element in a compound we can determine its formula. Convert grams aspirin to moles. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. the molecular mass of aspirin. Moles Aspirin Formula.
From www.chegg.com
Solved 9. Calculate the moles of aspirin in the sample Moles Aspirin Formula Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. There are two types of formulas, empirical and molecular. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Convert grams aspirin to moles. Molar mass of c9h8o4. Moles Aspirin Formula.
From www.numerade.com
SOLVED a) Calculate the number of moles of aspirin, C9H8O4, contained Moles Aspirin Formula knowing the mass of each element in a compound we can determine its formula. There are two types of formulas, empirical and molecular. In order to dissolve aspirin completely in water, you. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. Convert grams aspirin to moles. the molecular mass. Moles Aspirin Formula.
From www.coursehero.com
[Solved] From the reaction equation, stoichiometry of salicylic acid Moles Aspirin Formula molar mass of aspirin = 180.16 g. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. Convert grams aspirin to moles. the molecular mass of aspirin is (9 × 12) + (8 × 1) + (16 × 4) = 180 g. now that you know how many moles of aspirin you have in. Moles Aspirin Formula.
From www.numerade.com
SOLVED Stoichiometrlc Calculation Step? Mass of salicylic acid Moles Moles Aspirin Formula knowing the mass of each element in a compound we can determine its formula. There are two types of formulas, empirical and molecular. Molar mass of c9h8o4 = 180.15742 g/mol. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. In order to dissolve aspirin completely in water, you. Theoretical yield of aspirin (g) = (2. Moles Aspirin Formula.
From www.alamy.com
Fever reduce Black and White Stock Photos & Images Alamy Moles Aspirin Formula There are two types of formulas, empirical and molecular. Theoretical yield of aspirin (g) = (2 moles) (molar mass of aspirin) = 2.000 (180.16) = 360.3 grams. now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. Convert grams aspirin to moles. The mechanism of action. Moles Aspirin Formula.
From www.dreamstime.com
Acetylsalicylic Acid or Aspirin Drug Molecule. Skeletal Formula Stock Moles Aspirin Formula the chemical name of the active ingredient in aspirin is acetylsalicylic acid. molar mass of aspirin = 180.16 g. Convert grams aspirin to moles. There are two types of formulas, empirical and molecular. knowing the mass of each element in a compound we can determine its formula. Theoretical yield of aspirin (g) = (2 moles) (molar mass. Moles Aspirin Formula.
From taliyahtivecraig.blogspot.com
What Is the Mass of an Aspirin Tablet Moles Aspirin Formula now that you know how many moles of aspirin you have in your sample, use the fact that one mole of a substance. Molar mass of c9h8o4 = 180.15742 g/mol. the chemical name of the active ingredient in aspirin is acetylsalicylic acid. knowing the mass of each element in a compound we can determine its formula. The. Moles Aspirin Formula.