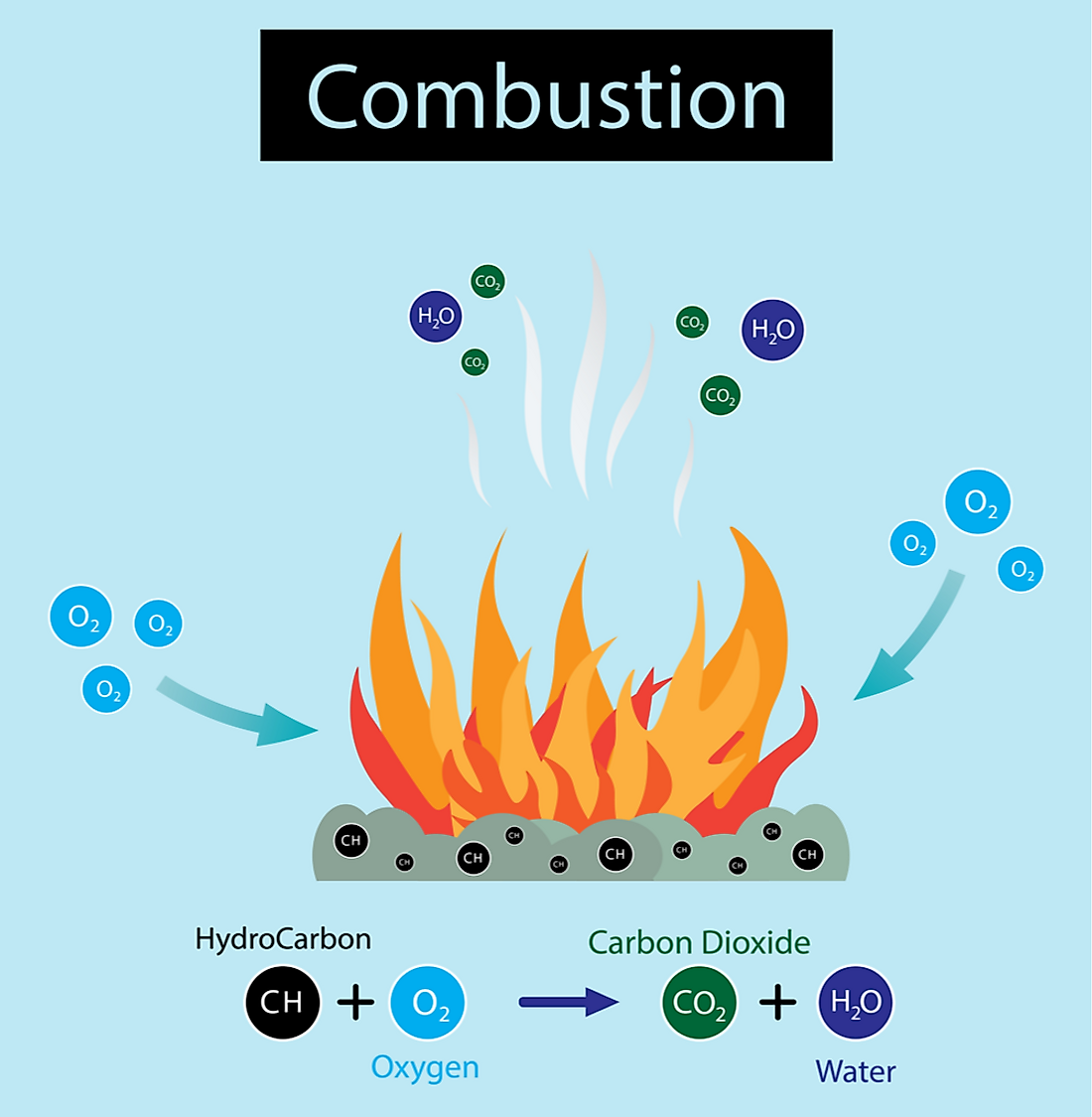
Does Fire Take Oxygen Out Of The Air . Oxygen supports the chemical processes that occur during fire. When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. However, oxygen is not the only oxidizing agent around. They quickly bond with oxygen in the air in a process called oxidation. By depriving a fire of air the fire can be. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. Flames are the visible portion of the fire, and it mainly consists of. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). But the atoms don’t stay single long: Unbound atoms form a hot gas, mingling with oxygen atoms in the air.
from www.worldatlas.com
They quickly bond with oxygen in the air in a process called oxidation. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). However, oxygen is not the only oxidizing agent around. By depriving a fire of air the fire can be. Oxygen supports the chemical processes that occur during fire. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. Flames are the visible portion of the fire, and it mainly consists of. When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. But the atoms don’t stay single long:
What Are The Properties Of Matter? WorldAtlas
Does Fire Take Oxygen Out Of The Air But the atoms don’t stay single long: Oxygen supports the chemical processes that occur during fire. They quickly bond with oxygen in the air in a process called oxidation. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. But the atoms don’t stay single long: By depriving a fire of air the fire can be. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. Flames are the visible portion of the fire, and it mainly consists of. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. However, oxygen is not the only oxidizing agent around.
From www.youtube.com
Air contains oxygen experiment Elementary Science YouTube Does Fire Take Oxygen Out Of The Air Flames are the visible portion of the fire, and it mainly consists of. But the atoms don’t stay single long: By depriving a fire of air the fire can be. However, oxygen is not the only oxidizing agent around. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of. Does Fire Take Oxygen Out Of The Air.
From www.researchgate.net
Photographs of the hydrogen flame, the propane flame, and the JetA Does Fire Take Oxygen Out Of The Air Unbound atoms form a hot gas, mingling with oxygen atoms in the air. When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. By depriving a fire of air the fire can be. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). However,. Does Fire Take Oxygen Out Of The Air.
From www.scienceabc.com
Can You Burn Water? Does Fire Take Oxygen Out Of The Air However, oxygen is not the only oxidizing agent around. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. By depriving a fire of air the fire can be. When fuel burns, it reacts. Does Fire Take Oxygen Out Of The Air.
From thehomeschoolscientist.com
Oxygen and Fire Experiment The Homeschool Scientist Does Fire Take Oxygen Out Of The Air Unbound atoms form a hot gas, mingling with oxygen atoms in the air. Flames are the visible portion of the fire, and it mainly consists of. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. In most cases, it is the ambient air, and in particular. Does Fire Take Oxygen Out Of The Air.
From www.alamy.com
Oxygen and fire science experiment illustration Stock Vector Image Does Fire Take Oxygen Out Of The Air Oxygen supports the chemical processes that occur during fire. By depriving a fire of air the fire can be. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. Flames are the visible portion of the fire, and it mainly consists of. However, oxygen is not the. Does Fire Take Oxygen Out Of The Air.
From www.slideserve.com
PPT Does fire need oxygen to burn? PowerPoint Presentation, free Does Fire Take Oxygen Out Of The Air But the atoms don’t stay single long: This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. Oxygen supports the chemical processes that occur during fire. They quickly bond with oxygen in the air in a process called oxidation. Flames are the visible portion of the fire,. Does Fire Take Oxygen Out Of The Air.
From www.tecservuk.com
What is the fire triangle? Tecserv UK Fire and Security Maintenance Does Fire Take Oxygen Out Of The Air However, oxygen is not the only oxidizing agent around. But the atoms don’t stay single long: Oxygen supports the chemical processes that occur during fire. They quickly bond with oxygen in the air in a process called oxidation. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. When fuel burns, it reacts with oxygen from the. Does Fire Take Oxygen Out Of The Air.
From thehomeschoolscientist.com
Oxygen and Fire Experiment The Homeschool Scientist Does Fire Take Oxygen Out Of The Air They quickly bond with oxygen in the air in a process called oxidation. When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. But the atoms don’t stay single long: Unbound atoms form a hot gas, mingling with oxygen atoms in the air. Oxygen supports the chemical processes that occur during fire.. Does Fire Take Oxygen Out Of The Air.
From www.slideserve.com
PPT Does fire need oxygen to burn? PowerPoint Presentation, free Does Fire Take Oxygen Out Of The Air Oxygen supports the chemical processes that occur during fire. By depriving a fire of air the fire can be. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. But the atoms don’t stay. Does Fire Take Oxygen Out Of The Air.
From www.slideserve.com
PPT Essentials of Fire Fighting , 5 th Edition PowerPoint Does Fire Take Oxygen Out Of The Air Unbound atoms form a hot gas, mingling with oxygen atoms in the air. However, oxygen is not the only oxidizing agent around. But the atoms don’t stay single long: This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. In most cases, it is the ambient air,. Does Fire Take Oxygen Out Of The Air.
From www.vedantu.com
Oxygen Cycle Learn Diagram, Importance, and Sources Does Fire Take Oxygen Out Of The Air When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. In most cases, it is the. Does Fire Take Oxygen Out Of The Air.
From www.alamy.com
Oxygen and fire science experiment illustration Stock Vector Image Does Fire Take Oxygen Out Of The Air When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). By. Does Fire Take Oxygen Out Of The Air.
From www.alamy.com
fire and oxygen. Science experiment with two candles and glass. Burning Does Fire Take Oxygen Out Of The Air Unbound atoms form a hot gas, mingling with oxygen atoms in the air. But the atoms don’t stay single long: Oxygen supports the chemical processes that occur during fire. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). When fuel burns, it reacts with oxygen from the surrounding air, releasing heat. Does Fire Take Oxygen Out Of The Air.
From www.shutterstock.com
Oxygen Needed Fire Burning Vector Diagram Stock Vector (Royalty Free Does Fire Take Oxygen Out Of The Air In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). Flames are the visible portion of the fire, and it mainly consists of. However, oxygen is not the only oxidizing agent around. They quickly bond with oxygen in the air in a process called oxidation. This glowing gas — and not the. Does Fire Take Oxygen Out Of The Air.
From www.slideserve.com
PPT chemistry of fire PowerPoint Presentation, free download ID1129778 Does Fire Take Oxygen Out Of The Air But the atoms don’t stay single long: Oxygen supports the chemical processes that occur during fire. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. Flames are the visible portion of the fire, and it mainly consists of. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at. Does Fire Take Oxygen Out Of The Air.
From leakdetection.co.uk
Fire Triangle Simple and Useful Guide Does Fire Take Oxygen Out Of The Air But the atoms don’t stay single long: By depriving a fire of air the fire can be. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). They quickly bond with oxygen in the air in a process called. Does Fire Take Oxygen Out Of The Air.
From www.dreamstime.com
Diagram of Oxygen and Fire Experiment Stock Vector Illustration of Does Fire Take Oxygen Out Of The Air Unbound atoms form a hot gas, mingling with oxygen atoms in the air. When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. They quickly bond with oxygen in the air in a process called oxidation. Flames are the visible portion of the fire, and it mainly consists of. But the atoms. Does Fire Take Oxygen Out Of The Air.
From www.alamy.com
Oxygen needed for fire and burning. vector diagram to demonstrate the Does Fire Take Oxygen Out Of The Air Oxygen supports the chemical processes that occur during fire. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. But the. Does Fire Take Oxygen Out Of The Air.
From www.youtube.com
Does Fire need Oxygen to Burn? I DIY Science Experiment for Kids YouTube Does Fire Take Oxygen Out Of The Air But the atoms don’t stay single long: When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). Unbound atoms form a hot gas, mingling with oxygen atoms in the air. By depriving a fire. Does Fire Take Oxygen Out Of The Air.
From www.slideserve.com
PPT Fire Hazards of Oxygen Enriched Atmospheres PowerPoint Does Fire Take Oxygen Out Of The Air They quickly bond with oxygen in the air in a process called oxidation. Oxygen supports the chemical processes that occur during fire. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). Unbound atoms form a hot gas, mingling with oxygen atoms in the air. This glowing gas — and not the. Does Fire Take Oxygen Out Of The Air.
From www.slideserve.com
PPT Fire Hazards of Oxygen Enriched Atmospheres PowerPoint Does Fire Take Oxygen Out Of The Air Oxygen supports the chemical processes that occur during fire. Flames are the visible portion of the fire, and it mainly consists of. But the atoms don’t stay single long: Unbound atoms form a hot gas, mingling with oxygen atoms in the air. By depriving a fire of air the fire can be. They quickly bond with oxygen in the air. Does Fire Take Oxygen Out Of The Air.
From www.slideserve.com
PPT Catching Killers PowerPoint Presentation ID2721332 Does Fire Take Oxygen Out Of The Air Flames are the visible portion of the fire, and it mainly consists of. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. They quickly bond with oxygen in the air in a process. Does Fire Take Oxygen Out Of The Air.
From northbridgefiredepartment.com
Don’t smoke around medical oxygen Northbridge Fire Department Does Fire Take Oxygen Out Of The Air They quickly bond with oxygen in the air in a process called oxidation. However, oxygen is not the only oxidizing agent around. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. Oxygen supports the chemical processes that occur. Does Fire Take Oxygen Out Of The Air.
From www.youtube.com
fire need oxygen to survive YouTube Does Fire Take Oxygen Out Of The Air When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. They quickly bond with oxygen in the air in a process called oxidation. Oxygen supports the chemical processes that occur during fire. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. But the atoms don’t stay single long:. Does Fire Take Oxygen Out Of The Air.
From www.worldatlas.com
What Are The Properties Of Matter? WorldAtlas Does Fire Take Oxygen Out Of The Air By depriving a fire of air the fire can be. But the atoms don’t stay single long: Flames are the visible portion of the fire, and it mainly consists of. When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. However, oxygen is not the only oxidizing agent around. This glowing gas. Does Fire Take Oxygen Out Of The Air.
From www.slideserve.com
PPT Fire Hazards of Oxygen Enriched Atmospheres PowerPoint Does Fire Take Oxygen Out Of The Air They quickly bond with oxygen in the air in a process called oxidation. In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). However, oxygen is not the only oxidizing agent around. Oxygen supports the chemical processes that occur during fire. When fuel burns, it reacts with oxygen from the surrounding air,. Does Fire Take Oxygen Out Of The Air.
From www.slideserve.com
PPT Hyperbaric Fire Fighting And Fire Prevention Procedures Does Fire Take Oxygen Out Of The Air Flames are the visible portion of the fire, and it mainly consists of. By depriving a fire of air the fire can be. Oxygen supports the chemical processes that occur during fire. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. But the atoms don’t stay. Does Fire Take Oxygen Out Of The Air.
From www.youtube.com
Does fire need air to burn Science Project Class 6 YouTube Does Fire Take Oxygen Out Of The Air In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). By depriving a fire of air the fire can be. Oxygen supports the chemical processes that occur during fire. However, oxygen is not the only oxidizing agent around. They quickly bond with oxygen in the air in a process called oxidation. When. Does Fire Take Oxygen Out Of The Air.
From www.slideserve.com
PPT chemistry of fire PowerPoint Presentation, free download ID1129778 Does Fire Take Oxygen Out Of The Air Flames are the visible portion of the fire, and it mainly consists of. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. This glowing gas — and not the fuel itself — produces the spooky blue light that. Does Fire Take Oxygen Out Of The Air.
From studylib.net
Does fire need oxygen to burn? Does Fire Take Oxygen Out Of The Air When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. By depriving a fire of air the fire can be. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the. Does Fire Take Oxygen Out Of The Air.
From www.grc.nasa.gov
Combustion Does Fire Take Oxygen Out Of The Air When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. They quickly bond with oxygen in the air in a process called oxidation. Oxygen supports the chemical processes that occur during fire. In most cases, it is the ambient. Does Fire Take Oxygen Out Of The Air.
From www.youtube.com
Do gas fireplaces take oxygen out of the air? YouTube Does Fire Take Oxygen Out Of The Air Oxygen supports the chemical processes that occur during fire. When fuel burns, it reacts with oxygen from the surrounding air, releasing heat and generating combustion products (gases,. Flames are the visible portion of the fire, and it mainly consists of. However, oxygen is not the only oxidizing agent around. They quickly bond with oxygen in the air in a process. Does Fire Take Oxygen Out Of The Air.
From www.ck12.org
Oxygen, Carbon Dioxide, and Energy CK12 Foundation Does Fire Take Oxygen Out Of The Air In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. Flames are the visible portion of the fire, and it mainly consists of. Oxygen supports the chemical processes that. Does Fire Take Oxygen Out Of The Air.
From www.cityfire.co.uk
Understanding The Fire Triangle Guide City Fire Protection Does Fire Take Oxygen Out Of The Air In most cases, it is the ambient air, and in particular one of its components, oxygen (o 2). Oxygen supports the chemical processes that occur during fire. Unbound atoms form a hot gas, mingling with oxygen atoms in the air. They quickly bond with oxygen in the air in a process called oxidation. However, oxygen is not the only oxidizing. Does Fire Take Oxygen Out Of The Air.
From wha-international.com
Real world oxygen fire evidence Oxygen fire investigations Does Fire Take Oxygen Out Of The Air By depriving a fire of air the fire can be. This glowing gas — and not the fuel itself — produces the spooky blue light that appears at the base of a flame. They quickly bond with oxygen in the air in a process called oxidation. Flames are the visible portion of the fire, and it mainly consists of. In. Does Fire Take Oxygen Out Of The Air.