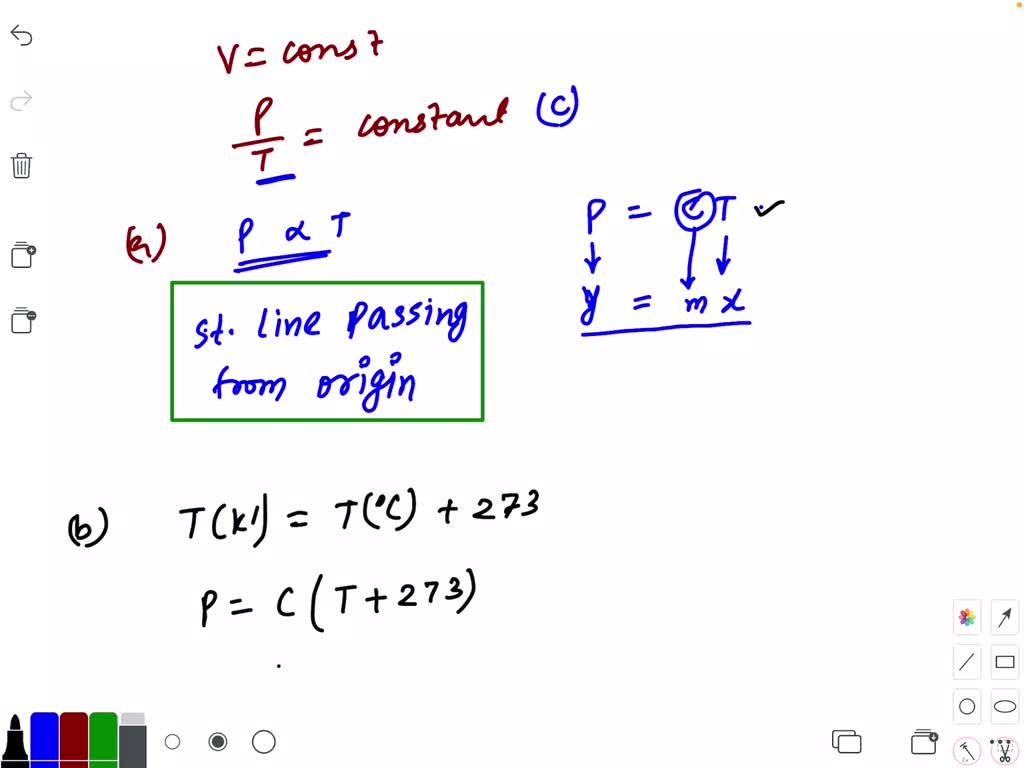
The Pressure And Absolute Temperature Of A Gas At Constant Volume Related . The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t.
from www.numerade.com
A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law).
SOLVEDThe pressure of a fixed quantity of gas at constant volume is
The Pressure And Absolute Temperature Of A Gas At Constant Volume Related A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law.
From www.doubtnut.com
The volumetemperature graphs of a given mass of an ideal gas at const The Pressure And Absolute Temperature Of A Gas At Constant Volume Related We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From byjus.com
7. The raise in the temperature of a given mass of an ideal gas at The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). We find that. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal The Pressure And Absolute Temperature Of A Gas At Constant Volume Related A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). In. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From sciencenotes.org
Boyle's Law Definition, Formula, Example The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From socratic.org
Which graph shows the relationship between the temperature and volume The Pressure And Absolute Temperature Of A Gas At Constant Volume Related A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law).. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.youtube.com
Pressure, Volume, Temperature and Mole Relationships YouTube The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. Charles's. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.grc.nasa.gov
Equation of State The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.nagwa.com
Question Video Finding the Pressure of a Gas Compressed over Constant The Pressure And Absolute Temperature Of A Gas At Constant Volume Related We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From slidetodoc.com
The relationship between temperature and volume How Volume The Pressure And Absolute Temperature Of A Gas At Constant Volume Related In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.toppr.com
Pressure versus temperature graph of an ideal gas at constant volume V The Pressure And Absolute Temperature Of A Gas At Constant Volume Related We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.slideserve.com
PPT Zeroth Law of Thermodynamics PowerPoint Presentation, free The Pressure And Absolute Temperature Of A Gas At Constant Volume Related We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial The Pressure And Absolute Temperature Of A Gas At Constant Volume Related Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. The ideal gas law can be derived from basic principles, but. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.britannica.com
Boyle’s law Definition, Equation, & Facts Britannica The Pressure And Absolute Temperature Of A Gas At Constant Volume Related We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. The ideal gas law can be derived from basic principles, but was originally deduced from. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas The Pressure And Absolute Temperature Of A Gas At Constant Volume Related A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. In. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.dreamstime.com
Boyle S Law, Relationship between Pressure and Volume of Gas at The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download The Pressure And Absolute Temperature Of A Gas At Constant Volume Related We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. Charles's law states that the volume of a given mass of gas varies directly with. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From owlcation.com
The Theories and Behavior of Gas Owlcation The Pressure And Absolute Temperature Of A Gas At Constant Volume Related Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. The volume of a given gas sample is directly proportional to its absolute temperature at constant. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.dreamstime.com
Boyle S Law Showing the Pressure and Volume Relationship Stock Vector The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. In. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From cartoondealer.com
Boyle's Law, Relationship Between Pressure And Volume Of Gas At The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.numerade.com
SOLVEDThe pressure of a fixed quantity of gas at constant volume is The Pressure And Absolute Temperature Of A Gas At Constant Volume Related In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. The volume of a given gas sample is directly proportional to. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.youtube.com
11.8 The Ideal Gas Law Pressure, Volume, Temperature, & Moles YouTube The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. In 1662, robert boyle systematically studied the relationship between the volume and pressure of. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From cartoondealer.com
Boyle's Law, Relationship Between Pressure And Volume Of Gas At The Pressure And Absolute Temperature Of A Gas At Constant Volume Related We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law.. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From owlcation.com
The Theories and Behavior of Gas Owlcation The Pressure And Absolute Temperature Of A Gas At Constant Volume Related We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. Charles's law states that the volume of a given mass of gas varies directly with. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.nagwa.com
Question Video Relating the Volume and Temperature of a Gas at The Pressure And Absolute Temperature Of A Gas At Constant Volume Related A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.youtube.com
Gas Pressure and Volume GCSE Physics Revision YouTube The Pressure And Absolute Temperature Of A Gas At Constant Volume Related In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.numerade.com
SOLVED Which of the following graphs shows the correct relationship The Pressure And Absolute Temperature Of A Gas At Constant Volume Related We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law.. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.numerade.com
SOLVED Which of the following represents the graph cf volume gas at The Pressure And Absolute Temperature Of A Gas At Constant Volume Related In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws The Pressure And Absolute Temperature Of A Gas At Constant Volume Related In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of charles’ law. A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.slideserve.com
PPT CHAPTER 12 GASES AND THEORY PowerPoint The Pressure And Absolute Temperature Of A Gas At Constant Volume Related A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. The ideal gas law can be derived from basic principles, but was originally deduced from experimental. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.toppr.com
The variation of coefficient of volume expansion of an ideal gas at The Pressure And Absolute Temperature Of A Gas At Constant Volume Related A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. Charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID The Pressure And Absolute Temperature Of A Gas At Constant Volume Related The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). A logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature.. The Pressure And Absolute Temperature Of A Gas At Constant Volume Related.