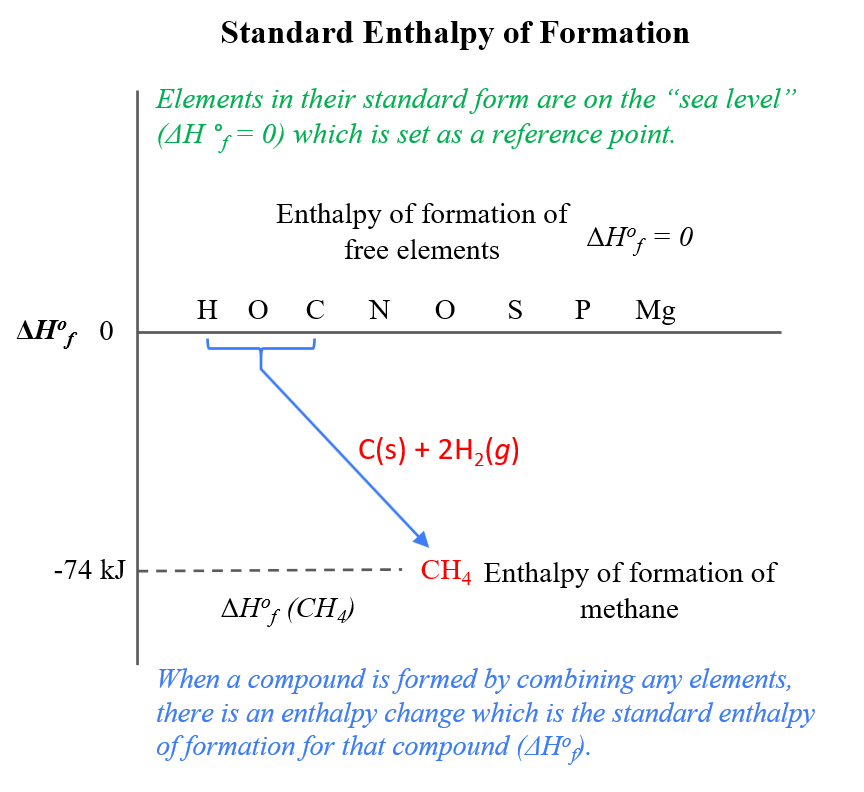
Standard Enthalpy Of Formation Vs Enthalpy Of Formation . enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign.
from exosbjzsg.blob.core.windows.net
enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature.
Standard Enthalpy Of Formation Al2O3 at Lois Woodburn blog
Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Vs Enthalpy Of Formation enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. the standard enthalpy of formation is. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.vrogue.co
Physical Chemistry 15 Enthalpy Change And Enthalpy Pr vrogue.co Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 Standard Enthalpy Of Formation Vs Enthalpy Of Formation by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From exosbjzsg.blob.core.windows.net
Standard Enthalpy Of Formation Al2O3 at Lois Woodburn blog Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. . Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube Standard Enthalpy Of Formation Vs Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Change YouTube Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the.. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Enthalpy Of Formation Vs Enthalpy Of Formation enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. by. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Vs Enthalpy Of Formation enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. the standard enthalpy. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.chegg.com
Solved The standard enthalpy (or heat) of reaction, delta H, Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy change is the enthalpy change that happens at standard pressure and. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID6642303 Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. by definition, the standard. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Chemistry 2e 5.3 YouTube Standard Enthalpy Of Formation Vs Enthalpy Of Formation enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation, δh. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From chemistry.stackexchange.com
thermodynamics Calculating Enthalpy of formation versus Calculating Enthalpy of a reaction not Standard Enthalpy Of Formation Vs Enthalpy Of Formation enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From pediaa.com
What is the Difference Between Enthalpy of Formation and Enthalpy of Reaction Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation,. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation* for Various Compounds Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From exoxhphey.blob.core.windows.net
Standard Enthalpy Of Formation Calculator at Susan blog Standard Enthalpy Of Formation Vs Enthalpy Of Formation by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Of Formation Vs Enthalpy Of Formation enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
How to Calculate the Standard Enthalpy of a Reaction from the Standard Enthalpies of Formation Standard Enthalpy Of Formation Vs Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From thechemistrynotes.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. the. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From chemistry.stackexchange.com
thermodynamics Calculating Enthalpy of formation versus Calculating Enthalpy of a reaction not Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. . Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy change is the enthalpy change that happens at standard pressure and. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. by definition, the standard enthalpy of formation of. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation is given the symbol δfho δ f h. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
6.6 Standard Enthalpy of Formation and Reaction YouTube Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is given the symbol δfho δ f h. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Vs Enthalpy Of Formation by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. enthalpies of formation. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From pediaa.com
What is the Difference Between Enthalpy of Formation and Enthalpy of Reaction Standard Enthalpy Of Formation Vs Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation Vs Enthalpy Of Formation by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Presentation ID5860500 Standard Enthalpy Of Formation Vs Enthalpy Of Formation by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign.. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
AP Chemistry Standard Enthalpies of Formation YouTube Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. . Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. by definition, the. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From www.youtube.com
Std Heat of Formation versus Bond Energy YouTube Standard Enthalpy Of Formation Vs Enthalpy Of Formation enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the. the standard enthalpy change is the enthalpy change that happens at standard pressure and temperature. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. 193 rows in chemistry. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From classzonemailmerge.z22.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy change is the enthalpy change that happens at standard pressure and. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.
From dxouglhlh.blob.core.windows.net
Standard Enthalpy Of Formation Mg at David Leon blog Standard Enthalpy Of Formation Vs Enthalpy Of Formation the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. enthalpies of formation measured under these conditions are called standard enthalpies of formation (δho f) the.. Standard Enthalpy Of Formation Vs Enthalpy Of Formation.