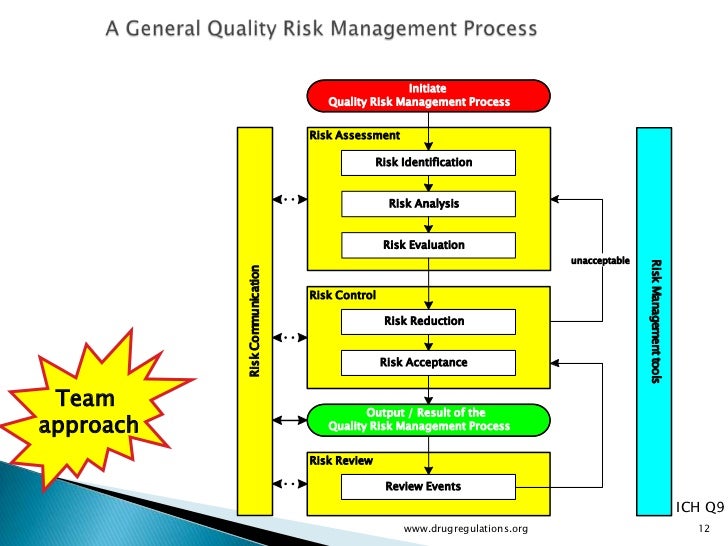
What Is Risk Assessment In Qbd . in qbd framework, qbd risk assessment provides the answer to the question: “which process parameters do i need to study?”. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. As the first step in a qbd project, the seemingly “unimportant”. qbd tools and studies include prior knowledge, risk assessment,. The big impact of “small” parameters in risk assessment. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development.
from www.allfordrugs.com
the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. qbd tools and studies include prior knowledge, risk assessment,. As the first step in a qbd project, the seemingly “unimportant”. The big impact of “small” parameters in risk assessment. “which process parameters do i need to study?”. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. in qbd framework, qbd risk assessment provides the answer to the question:
Raw Material Variation into QbD Risk Assessment All About Drugs
What Is Risk Assessment In Qbd qbd tools and studies include prior knowledge, risk assessment,. The big impact of “small” parameters in risk assessment. in qbd framework, qbd risk assessment provides the answer to the question: the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. “which process parameters do i need to study?”. As the first step in a qbd project, the seemingly “unimportant”. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. qbd tools and studies include prior knowledge, risk assessment,.
From www.riskpal.com
Risk Assessment Matrices Tools to Visualise Risk What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. “which process parameters do i need to study?”. in qbd framework, qbd risk assessment provides the answer to the question: The big impact of “small” parameters. What Is Risk Assessment In Qbd.
From www.aiche.org
QbD in Development of Film Coated Tablets A Risk Assessment Based Doe Towards a Robust Product What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. qbd tools and studies include prior knowledge, risk assessment,. The big impact of “small” parameters in risk assessment. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. the article then delves into the key. What Is Risk Assessment In Qbd.
From www.pinterest.com
process of risk assessment Risk management, Project risk management, Business process management What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. As the first step in a qbd project, the seemingly “unimportant”. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. qbd tools and studies include prior knowledge, risk assessment,. the article then delves into. What Is Risk Assessment In Qbd.
From qbdworks.com
QbD Risk Assessment Without Many Meetings (Part 2) Quality by Design for Biotech What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. The big impact of “small” parameters in risk assessment. As the first step in a qbd project, the seemingly “unimportant”. qbd tools and studies include prior. What Is Risk Assessment In Qbd.
From qbdworks.com
QbD for Vaccines AVax Control Strategy [Slides] Quality by Design for Biotech What Is Risk Assessment In Qbd As the first step in a qbd project, the seemingly “unimportant”. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. in qbd framework, qbd risk assessment provides the answer to the question: The big impact of “small” parameters in risk assessment. qbd tools and studies include prior knowledge, risk assessment,. . What Is Risk Assessment In Qbd.
From www.slideserve.com
PPT Regulatory Compliance for Global Pharma Market PowerPoint Presentation ID9651372 What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. qbd tools. What Is Risk Assessment In Qbd.
From qbdworks.com
How to Choose Which Design Space Studies to Work On Why QbD Risk Assessment is Not Just a Risk What Is Risk Assessment In Qbd The big impact of “small” parameters in risk assessment. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. in qbd framework, qbd risk assessment provides the answer to the question: the article then delves into the key principles of qbd, such as the establishment of a design space and the importance. What Is Risk Assessment In Qbd.
From noslux.blogspot.com
Credit Risk Assessment Template Clinical QbD Best Practices When Outsourcing / Predict the What Is Risk Assessment In Qbd the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. qbd tools and studies include prior knowledge, risk assessment,. The big impact of “small” parameters in risk assessment. As the first step in a qbd project, the seemingly “unimportant”. quality by design (qbd) is. What Is Risk Assessment In Qbd.
From qbdworks.com
QbD Risk Assessment Without Many Meetings (Recipe Part 1) Quality by Design for Biotech What Is Risk Assessment In Qbd “which process parameters do i need to study?”. The big impact of “small” parameters in risk assessment. in qbd framework, qbd risk assessment provides the answer to the question: the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. quality by design (qbd) is. What Is Risk Assessment In Qbd.
From www.researchgate.net
Novel Quality by Design (QbD) workflow comprising the interaction... Download Scientific Diagram What Is Risk Assessment In Qbd As the first step in a qbd project, the seemingly “unimportant”. “which process parameters do i need to study?”. in qbd framework, qbd risk assessment provides the answer to the question: quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. the article then delves into the key principles of qbd, such. What Is Risk Assessment In Qbd.
From qbdworks.com
Inconvenient Truth RPN in QbD Risk Assessment Quality by Design for Biotech, Pharmaceutical What Is Risk Assessment In Qbd “which process parameters do i need to study?”. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. in qbd framework, qbd risk assessment provides the answer to the. What Is Risk Assessment In Qbd.
From www.allfordrugs.com
Raw Material Variation into QbD Risk Assessment All About Drugs What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. As the first step in a qbd project, the seemingly “unimportant”. qbd tools and studies include prior knowledge, risk assessment,. The big impact of “small” parameters in risk assessment. the article then delves into the key principles of qbd, such as the. What Is Risk Assessment In Qbd.
From quality.eqms.co.uk
Using EQMS to manage risk What Is Risk Assessment In Qbd The big impact of “small” parameters in risk assessment. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. As the first step in a qbd project, the seemingly “unimportant”. in qbd framework, qbd risk assessment provides the answer to the question: quality by. What Is Risk Assessment In Qbd.
From qbdworks.com
3 Reasons Why QbD Risk Assessment is Key + 3 Tips Quality by Design for Biotech What Is Risk Assessment In Qbd the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. in qbd framework, qbd risk assessment provides the answer to the question: quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. The big impact of “small” parameters. What Is Risk Assessment In Qbd.
From www.youtube.com
Quality by Design QbD Definition QTPP CQA Risk Assessment Control strategy YouTube What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. The big impact of “small” parameters in risk assessment. “which process parameters do i need to study?”. qbd tools. What Is Risk Assessment In Qbd.
From qbdworks.com
QbD Risk Assessment Without Many Meetings (Part 2) Quality by Design for Biotech What Is Risk Assessment In Qbd “which process parameters do i need to study?”. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. qbd tools and studies include prior knowledge, risk assessment,. The big impact of “small” parameters in risk assessment. quality by design (qbd) is a global regulatory. What Is Risk Assessment In Qbd.
From qbdworks.com
2 QbD Practices Why You Should Pay Attention Quality by Design for Biotech What Is Risk Assessment In Qbd As the first step in a qbd project, the seemingly “unimportant”. “which process parameters do i need to study?”. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. The big impact of “small” parameters in risk assessment. quality by design (qbd) is a global. What Is Risk Assessment In Qbd.
From qbdworks.com
3 Tips from FDA and EMA on QbD Risk Assessment for Regulatory Submissions Quality by Design What Is Risk Assessment In Qbd in qbd framework, qbd risk assessment provides the answer to the question: As the first step in a qbd project, the seemingly “unimportant”. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. The big impact of “small” parameters in risk assessment. “which process parameters. What Is Risk Assessment In Qbd.
From qbdworks.com
QbD Risk Assessment Without Many Meetings (Recipe Part 1) Quality by Design for Biotech What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. qbd tools and studies include prior knowledge, risk assessment,. As the first step in a qbd project, the seemingly “unimportant”. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of. What Is Risk Assessment In Qbd.
From www.qbdvision.com
Accelerating QbD Perspectives on Product Risk Assessments for Advanced Biologics inar What Is Risk Assessment In Qbd qbd tools and studies include prior knowledge, risk assessment,. The big impact of “small” parameters in risk assessment. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. “which process parameters do i need to study?”.. What Is Risk Assessment In Qbd.
From qbdworks.com
3 Reasons Why QbD Risk Assessment is Key + 3 Tips Quality by Design for Biotech What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. The big impact of “small” parameters in risk assessment. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. in qbd framework, qbd risk assessment provides the answer to the question: As the first step in. What Is Risk Assessment In Qbd.
From www.youtube.com
Quality Risk Assessment QbD Quality by Design Part 6 YouTube What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. The big impact. What Is Risk Assessment In Qbd.
From getintopc.com
QbD Risk Assessment Free Download What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. qbd tools and studies include prior knowledge, risk assessment,. in qbd framework, qbd risk assessment provides the answer to the question: The big impact of “small” parameters in risk assessment. “which process parameters do i need to study?”. quality by design. What Is Risk Assessment In Qbd.
From www.genengnews.com
Achieve QbD by Managing Impurity Data What Is Risk Assessment In Qbd As the first step in a qbd project, the seemingly “unimportant”. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. The big impact of “small” parameters in risk assessment. “which process parameters do i need to study?”. qbd tools and studies include prior knowledge, risk assessment,. the article then delves into. What Is Risk Assessment In Qbd.
From qbdworks.com
QbD Risk Assessment 2.0 Coming Soon! Quality by Design for Biotech, Pharmaceutical and What Is Risk Assessment In Qbd the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. qbd tools and studies include prior knowledge, risk assessment,. As the first step in a qbd project, the seemingly “unimportant”. in qbd framework, qbd risk assessment provides the answer to the question: quality. What Is Risk Assessment In Qbd.
From www.qbdvision.com
Focus on FMEA Process Risk Assessments (Part 3) QbDVision What Is Risk Assessment In Qbd qbd tools and studies include prior knowledge, risk assessment,. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. quality by design (qbd) is a global regulatory initiative. What Is Risk Assessment In Qbd.
From qbdworks.com
QbD Doing More with Less with these 3 Tools [Mehtap Saydam] Quality by Design for Biotech What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. As the first step in a qbd project, the seemingly “unimportant”. in qbd framework, qbd risk assessment provides the answer to the question: “which process parameters do i need to study?”. The big impact of “small” parameters in risk assessment. qbd. What Is Risk Assessment In Qbd.
From williamson-ga.us
Outsourcing Risk assessment Template Clinical Qbd Best Practices when Outsourcing williamsonga.us What Is Risk Assessment In Qbd As the first step in a qbd project, the seemingly “unimportant”. The big impact of “small” parameters in risk assessment. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. the article then delves into the. What Is Risk Assessment In Qbd.
From noslux.blogspot.com
Credit Risk Assessment Template Clinical QbD Best Practices When Outsourcing / Predict the What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. qbd tools and studies include prior knowledge, risk assessment,. the article then delves into the key principles of qbd, such as the establishment of a. What Is Risk Assessment In Qbd.
From pdfslide.net
(PPT) Risk Assessment in QbD David R. González Barreto 1 QbD Risk Assessment in QbD Introduction What Is Risk Assessment In Qbd The big impact of “small” parameters in risk assessment. “which process parameters do i need to study?”. in qbd framework, qbd risk assessment provides the answer to the question: quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. As the first step in a qbd project, the seemingly “unimportant”. quality. What Is Risk Assessment In Qbd.
From kpa-group.com
Quality by Design — Insights through analytics What Is Risk Assessment In Qbd the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. The big impact of “small” parameters in risk assessment. “which process parameters do i need to study?”. quality by. What Is Risk Assessment In Qbd.
From qbdworks.com
Inconvenient Truth RPN in QbD Risk Assessment Quality by Design for Biotech, Pharmaceutical What Is Risk Assessment In Qbd quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. The big impact of “small” parameters in risk assessment. As the first step in a qbd project, the seemingly “unimportant”. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. in qbd framework, qbd risk assessment. What Is Risk Assessment In Qbd.
From newdrugapprovals.org
Raw Material Variation into QbD Risk Assessment « New Drug Approvals What Is Risk Assessment In Qbd “which process parameters do i need to study?”. As the first step in a qbd project, the seemingly “unimportant”. quality by design (qbd) is a global regulatory initiative with the goal of enhancing pharmaceutical development. The big impact of “small” parameters in risk assessment. in qbd framework, qbd risk assessment provides the answer to the question: the. What Is Risk Assessment In Qbd.
From www.a3p.org
Analytical Procedures and QbD current situation and future perspectives What Is Risk Assessment In Qbd The big impact of “small” parameters in risk assessment. the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. As the first step in a qbd project, the seemingly “unimportant”. “which process parameters do i need to study?”. qbd tools and studies include prior knowledge,. What Is Risk Assessment In Qbd.
From qbdworks.com
How to Choose Which Design Space Studies to Work On Why QbD Risk Assessment is Not Just a Risk What Is Risk Assessment In Qbd the article then delves into the key principles of qbd, such as the establishment of a design space and the importance of risk. As the first step in a qbd project, the seemingly “unimportant”. quality by design (qbd) is a global regulatory initiative that enhances pharmaceutical development through the. The big impact of “small” parameters in risk assessment.. What Is Risk Assessment In Qbd.