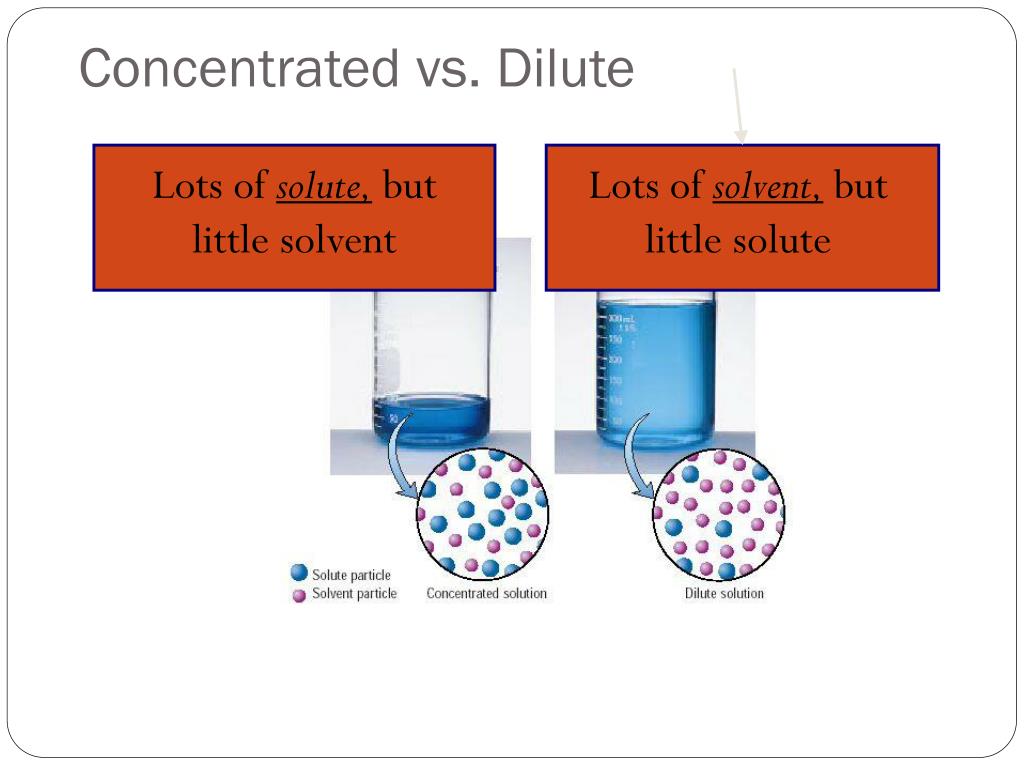
What Is Dilution Water . We are often concerned with how much solute is dissolved in a given amount of. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Concentration is the removal of solvent, which increases the. For example, if you have a glass. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. In both dilution and concentration,. The diluted liquid needs to be thoroughly mixed to achieve. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.
from www.slideserve.com
Understand how stock solutions are used in the laboratory. For example, if you have a glass. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). We are often concerned with how much solute is dissolved in a given amount of. In both dilution and concentration,. The diluted liquid needs to be thoroughly mixed to achieve. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent.
PPT Water and the aqueous systems PowerPoint Presentation, free
What Is Dilution Water Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the. We are often concerned with how much solute is dissolved in a given amount of. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. For example, if you have a glass. The diluted liquid needs to be thoroughly mixed to achieve. Understand how stock solutions are used in the laboratory. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions What Is Dilution Water The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Concentration is the removal of solvent, which increases the. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. For example, if you have a glass. Understand how. What Is Dilution Water.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians What Is Dilution Water Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. We are often concerned with how much solute is dissolved in a given amount of. The dilution ratio is the ratio of the solute (the substance to be diluted) to. What Is Dilution Water.
From letstalkscience.ca
Osmosis and Its Role in Human Biology and Health Let's Talk Science What Is Dilution Water In both dilution and concentration,. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like. What Is Dilution Water.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory What Is Dilution Water Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions. What Is Dilution Water.
From www.slideserve.com
PPT What is Dilution?? PowerPoint Presentation, free download ID What Is Dilution Water The diluted liquid needs to be thoroughly mixed to achieve. For example, if you have a glass. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the. What Is Dilution Water.
From www.weberscientific.com
er Scientific Buffered Phosphate Dilution Water What Is Dilution Water Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. In both dilution and concentration,. The diluted liquid needs to be thoroughly mixed to achieve. We are often concerned with. What Is Dilution Water.
From slideplayer.com
Dissolved Oxygen and Biochemical Oxygen Demand Analyses ppt download What Is Dilution Water The diluted liquid needs to be thoroughly mixed to achieve. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with. What Is Dilution Water.
From www.teachoo.com
Assertion (A) To dilute sulphuric acid, acid is added to water and n What Is Dilution Water We are often concerned with how much solute is dissolved in a given amount of. Concentration is the removal of solvent, which increases the. For example, if you have a glass. Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The dilution ratio is. What Is Dilution Water.
From www.vrogue.co
10 Examples Of Solutions vrogue.co What Is Dilution Water Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Concentration is the removal of solvent, which increases the. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Dilution is the addition of solvent, which decreases the. What Is Dilution Water.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo What Is Dilution Water The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Concentration is the removal of solvent, which increases the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Dilution is the process of decreasing the concentration of a solute. What Is Dilution Water.
From www.youtube.com
Dilution Method Problems Waste Water Engineering YouTube What Is Dilution Water The diluted liquid needs to be thoroughly mixed to achieve. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. For example, if you have a glass. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. The dilution ratio. What Is Dilution Water.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of What Is Dilution Water The diluted liquid needs to be thoroughly mixed to achieve. In both dilution and concentration,. Concentration is the removal of solvent, which increases the. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. The dilution ratio is the ratio of the solute (the substance to be. What Is Dilution Water.
From www.actioncleanup.com
Dilution Action’s Guide to Mixing the Right Solutions What Is Dilution Water Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Concentration is. What Is Dilution Water.
From www.slideserve.com
PPT To understand the process of dissolving To learn why certain What Is Dilution Water Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. We are often concerned with how much solute is dissolved in a given amount of. Concentration is the removal of solvent, which increases the. For example, if you have a glass. The dilution ratio is the ratio. What Is Dilution Water.
From extension.uga.edu
Understanding Laboratory Wastewater Tests I. Organics (BOD, COD, TOC What Is Dilution Water Understand how stock solutions are used in the laboratory. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the. What Is Dilution Water.
From carlosgokeowen.blogspot.com
What is Dilution What Is Dilution Water In both dilution and concentration,. The diluted liquid needs to be thoroughly mixed to achieve. Concentration is the removal of solvent, which increases the. We are often concerned with how much solute is dissolved in a given amount of. Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the. What Is Dilution Water.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts What Is Dilution Water Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. The diluted liquid needs to be thoroughly mixed to achieve. Understand how stock solutions are used in the laboratory. In. What Is Dilution Water.
From www.mpimpex.co.th
eZy™ Butterfield's PhosphateBuffered Dilution Water (pH 7.2), 90450 What Is Dilution Water Concentration is the removal of solvent, which increases the. The diluted liquid needs to be thoroughly mixed to achieve. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. We are often. What Is Dilution Water.
From zhtutorials.com
Serial Dilution Practical Skills Ep 3 Zoë Huggett Tutorials What Is Dilution Water For example, if you have a glass. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. In both dilution and concentration,. Concentration is the removal. What Is Dilution Water.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture What Is Dilution Water For example, if you have a glass. Concentration is the removal of solvent, which increases the. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). In both dilution and concentration,. The diluted liquid needs to be thoroughly mixed to achieve. Understand how stock solutions are used in the laboratory. Dilution. What Is Dilution Water.
From albertawater.com
Alberta WaterPortal Dilution Alberta WaterPortal What Is Dilution Water Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. For example, if you have a glass. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases the concentration of the solute. What Is Dilution Water.
From www.researchgate.net
Characteristics of dilution water Download Table What Is Dilution Water For example, if you have a glass. In both dilution and concentration,. The diluted liquid needs to be thoroughly mixed to achieve. Concentration is the removal of solvent, which increases the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of decreasing the concentration of a solute in a. What Is Dilution Water.
From carlosgokeowen.blogspot.com
What is Dilution What Is Dilution Water Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The diluted liquid needs to be thoroughly mixed to achieve. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Concentration is the removal of solvent, which increases the. Understand how stock solutions are used in the. What Is Dilution Water.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The What Is Dilution Water We are often concerned with how much solute is dissolved in a given amount of. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). For example, if you have a glass. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with. What Is Dilution Water.
From studymarxianism.z21.web.core.windows.net
How To Dilute Concentrated Acid What Is Dilution Water The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the process of decreasing the concentration of a. What Is Dilution Water.
From www.slideserve.com
PPT Water and the aqueous systems PowerPoint Presentation, free What Is Dilution Water The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Concentration is the removal of solvent, which increases the. Understand how stock solutions are used in the laboratory. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the process of making a solution. What Is Dilution Water.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts What Is Dilution Water Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. In both dilution and concentration,. Understand how stock solutions are used in the laboratory. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Dilution is the addition. What Is Dilution Water.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The What Is Dilution Water In both dilution and concentration,. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). We are often concerned with how much solute is dissolved in a given amount of. The diluted liquid needs to be thoroughly mixed to achieve. Dilution is the addition of solvent, which decreases the concentration of. What Is Dilution Water.
From www.wikihow.com
ImageDilute an Acid Step 12.jpg wikiHow What Is Dilution Water Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Understand how stock solutions are used in the laboratory. Dilution is the process of decreasing the concentration of a solute. What Is Dilution Water.
From www.researchgate.net
Characteristics of Dilution Water Download Scientific Diagram What Is Dilution Water The diluted liquid needs to be thoroughly mixed to achieve. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Understand how stock solutions are used in the laboratory. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the. What Is Dilution Water.
From www.slideserve.com
PPT Molarity and Dilutions PowerPoint Presentation, free download What Is Dilution Water Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. In both dilution and concentration,.. What Is Dilution Water.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation, free What Is Dilution Water Concentration is the removal of solvent, which increases the. For example, if you have a glass. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Dilution is the process of decreasing the concentration. What Is Dilution Water.
From www.researchgate.net
Exactly why do we dilute waste water samples to find BOD? and how to What Is Dilution Water The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Concentration is the removal of solvent, which increases the. We are. What Is Dilution Water.
From sonia-has-mcneil.blogspot.com
Describe How Dilution Was Used to Prepare the Starch Solutions Sonia What Is Dilution Water Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. We are often concerned with how much solute is dissolved in a given amount of. Concentration is the removal of solvent, which increases the. For example, if you have a glass. Understand how stock solutions are used in the laboratory. The diluted liquid. What Is Dilution Water.
From sciencenotes.org
Add Acid to Water or Water to Acid? Safely Diluting Acids What Is Dilution Water Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding. We are often concerned with how much solute is dissolved in a given amount of. The diluted liquid needs to be. What Is Dilution Water.