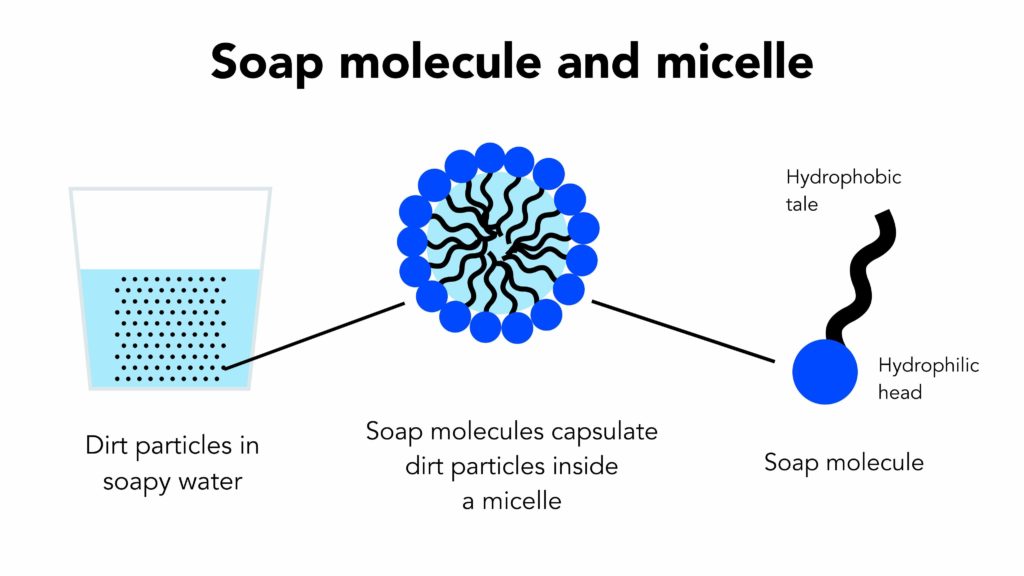
Soap Chemistry Hydrophobic . If the ph of a soap solution is lowered by acidic. soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances. The hydrophobic tail, derived from the fatty acid component,. how soap works is due to its unique chemistry, the hydrophilic (loves water). soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap.
from ar.inspiredpencil.com
The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances. The hydrophobic tail, derived from the fatty acid component,. how soap works is due to its unique chemistry, the hydrophilic (loves water). soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. If the ph of a soap solution is lowered by acidic. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap.
Soap Molecule Structure
Soap Chemistry Hydrophobic how soap works is due to its unique chemistry, the hydrophilic (loves water). If the ph of a soap solution is lowered by acidic. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances. The hydrophobic tail, derived from the fatty acid component,. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. how soap works is due to its unique chemistry, the hydrophilic (loves water).
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Soap Chemistry Hydrophobic soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. If the ph of a soap solution is lowered by acidic. The hydrophobic tail, derived from the fatty acid component,. how soap works is due to its unique chemistry, the hydrophilic (loves water). The other end of the molecule is a nonpolar chain of fatty acids. Soap Chemistry Hydrophobic.
From www.pinterest.com
How Soap Work? Soap, Cleanse, Basic concepts Soap Chemistry Hydrophobic soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. how soap works is due to its unique chemistry, the hydrophilic (loves water). The hydrophobic tail, derived from the. Soap Chemistry Hydrophobic.
From dxoqrmmqx.blob.core.windows.net
Liquid Soap Chemical Formula at Walter Battle blog Soap Chemistry Hydrophobic how soap works is due to its unique chemistry, the hydrophilic (loves water). The hydrophobic tail, derived from the fatty acid component,. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. soap and detergent,. Soap Chemistry Hydrophobic.
From www.shutterstock.com
Hydrophobic End Soap Molecules Interacts Dirt เวกเตอร์สต็อก (ปลอดค่า Soap Chemistry Hydrophobic soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. how soap works is due to its unique chemistry, the hydrophilic (loves water). grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. If the ph of a soap solution is lowered by acidic. solutions. Soap Chemistry Hydrophobic.
From aliceinchemiland.blogspot.com
Organic Chemistry in My Daily Life Organic Chemistry about Soap and Soap Chemistry Hydrophobic If the ph of a soap solution is lowered by acidic. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. how soap works is due to its unique chemistry, the hydrophilic (loves water). grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. soap. Soap Chemistry Hydrophobic.
From ar.inspiredpencil.com
Soap Molecule Structure Soap Chemistry Hydrophobic soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. If the ph of a soap solution is lowered by acidic. The hydrophobic tail, derived from the fatty acid component,. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. grease and. Soap Chemistry Hydrophobic.
From www.thoughtco.com
How Soap Works Soap Chemistry Hydrophobic soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water. Soap Chemistry Hydrophobic.
From www.themacbath.com
Back to Basics What Is Soap and How Does It Work? — The MacBath Soap Chemistry Hydrophobic The hydrophobic tail, derived from the fatty acid component,. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances.. Soap Chemistry Hydrophobic.
From cosmosmagazine.com
The chemistry of soap Soap Chemistry Hydrophobic grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. The hydrophobic tail, derived from the fatty acid component,. how soap works is due to its unique chemistry, the hydrophilic (loves water). soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. solutions of alkali. Soap Chemistry Hydrophobic.
From myluvlychemistry.blogspot.com
my chemistry Cleansing Action Of Soap and Detergent Soap Chemistry Hydrophobic how soap works is due to its unique chemistry, the hydrophilic (loves water). grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. If the ph of a soap solution is lowered by acidic. soap. Soap Chemistry Hydrophobic.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 Soap Chemistry Hydrophobic The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances. soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. how soap works is due to its unique. Soap Chemistry Hydrophobic.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab Soap Chemistry Hydrophobic The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances. solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. soap and detergent, substances that, when dissolved in water, possess the ability. Soap Chemistry Hydrophobic.
From www.youtube.com
10th chemistry cleansing action of soap ,hydrophobic,hydrophilic in Soap Chemistry Hydrophobic If the ph of a soap solution is lowered by acidic. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted. Soap Chemistry Hydrophobic.
From slideplayer.com
Importance of Chemistry in Your daily life ppt download Soap Chemistry Hydrophobic how soap works is due to its unique chemistry, the hydrophilic (loves water). solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. The other end of the molecule is a nonpolar chain. Soap Chemistry Hydrophobic.
From www.alamy.com
When dissolved in water, fatty acids in soap form micelles, the Soap Chemistry Hydrophobic solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. how soap works is due to its unique chemistry, the hydrophilic (loves water). The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily. Soap Chemistry Hydrophobic.
From www.slideshare.net
Chemistry of soaps Soap Chemistry Hydrophobic soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. grease and oil droplets are solubilized in water when they. Soap Chemistry Hydrophobic.
From exoxmnoxz.blob.core.windows.net
How Does Soap Work Science at Kelly Kline blog Soap Chemistry Hydrophobic how soap works is due to its unique chemistry, the hydrophilic (loves water). The hydrophobic tail, derived from the fatty acid component,. soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from. Soap Chemistry Hydrophobic.
From www.youtube.com
Hydrolysis & How It Is Used To Make Soaps Organic Chemistry Soap Chemistry Hydrophobic grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances. The hydrophobic tail, derived from the fatty acid component,.. Soap Chemistry Hydrophobic.
From news.mit.edu
Using soap to remove micropollutants from water MIT News Soap Chemistry Hydrophobic grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. how soap works is due to its unique chemistry, the hydrophilic (loves water). The hydrophobic tail, derived from the fatty acid component,. If the ph of a soap solution is lowered by acidic. soap molecules are amphiphilic, meaning. Soap Chemistry Hydrophobic.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID Soap Chemistry Hydrophobic how soap works is due to its unique chemistry, the hydrophilic (loves water). soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. soap and detergent, substances that, when dissolved in water, possess the ability. Soap Chemistry Hydrophobic.
From pubs.sciepub.com
Figure 2. Structure of a soap bubble and a soap film Practical Soap Chemistry Hydrophobic The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. The hydrophobic tail, derived from. Soap Chemistry Hydrophobic.
From www.numerade.com
SOLVEDSoap molecules have a hydrophobic portion and yet they dissolve Soap Chemistry Hydrophobic soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. how soap works is due to. Soap Chemistry Hydrophobic.
From saylordotorg.github.io
Aggregate Particles in Aqueous Solution Soap Chemistry Hydrophobic solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. The hydrophobic tail, derived from the fatty acid component,. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances. soap molecules have. Soap Chemistry Hydrophobic.
From www.britannica.com
Emulsion polymerization chemistry Britannica Soap Chemistry Hydrophobic The hydrophobic tail, derived from the fatty acid component,. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap.. Soap Chemistry Hydrophobic.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 Soap Chemistry Hydrophobic If the ph of a soap solution is lowered by acidic. solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. how soap works is due to its unique chemistry, the hydrophilic (loves water). grease and oil droplets are. Soap Chemistry Hydrophobic.
From www.youtube.com
Chemistry 101 How does soap work? YouTube Soap Chemistry Hydrophobic If the ph of a soap solution is lowered by acidic. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which. Soap Chemistry Hydrophobic.
From aliceinchemiland.blogspot.com
Organic Chemistry in My Daily Life Organic Chemistry about Soap and Soap Chemistry Hydrophobic The hydrophobic tail, derived from the fatty acid component,. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which is hydrophobic—meaning that it’s repelled by water but attracted to grease and other oily substances. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. grease and oil droplets are solubilized. Soap Chemistry Hydrophobic.
From slideplayer.com
Micelles to the Rescue How Soap Transports Debris ppt download Soap Chemistry Hydrophobic The hydrophobic tail, derived from the fatty acid component,. solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. If the ph of a soap solution is lowered by acidic. grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. how soap works. Soap Chemistry Hydrophobic.
From stock.adobe.com
Surfactant removing dirt from skin surface, surfactants, soap, solution Soap Chemistry Hydrophobic soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. how soap works is due to its unique chemistry, the hydrophilic (loves water). If the ph of a soap solution is lowered by. Soap Chemistry Hydrophobic.
From cejagubm.blob.core.windows.net
How Does Soap Work To Reduce Microbes In Chemistry Of Soap at Anna Soap Chemistry Hydrophobic how soap works is due to its unique chemistry, the hydrophilic (loves water). The hydrophobic tail, derived from the fatty acid component,. soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from. Soap Chemistry Hydrophobic.
From www.vrogue.co
Draw Labelled Diagram Of Soap Micelle Chemistry Shaal vrogue.co Soap Chemistry Hydrophobic If the ph of a soap solution is lowered by acidic. solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. The other end of the molecule is a nonpolar. Soap Chemistry Hydrophobic.
From saylordotorg.github.io
Solutions Soap Chemistry Hydrophobic soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. The hydrophobic tail, derived from the fatty acid component,. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. If the ph of a soap solution is lowered by acidic. grease and. Soap Chemistry Hydrophobic.
From thesoapmoleculeco.com
The Soap Molecule Co. Soap Chemistry Hydrophobic how soap works is due to its unique chemistry, the hydrophilic (loves water). If the ph of a soap solution is lowered by acidic. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. soap molecules have on one end what’s known as a. Soap Chemistry Hydrophobic.
From www.slideshare.net
Soap and detergent, medicine , food additives consumer 2011edited2 Soap Chemistry Hydrophobic how soap works is due to its unique chemistry, the hydrophilic (loves water). soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. The hydrophobic. Soap Chemistry Hydrophobic.
From www.alamy.com
General formula of solid soap molecule. Sodium carboxylate, RCOONa. It Soap Chemistry Hydrophobic grease and oil droplets are solubilized in water when they are coated by the nonpolar, hydrophobic tails of soap. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons, which. Soap Chemistry Hydrophobic.