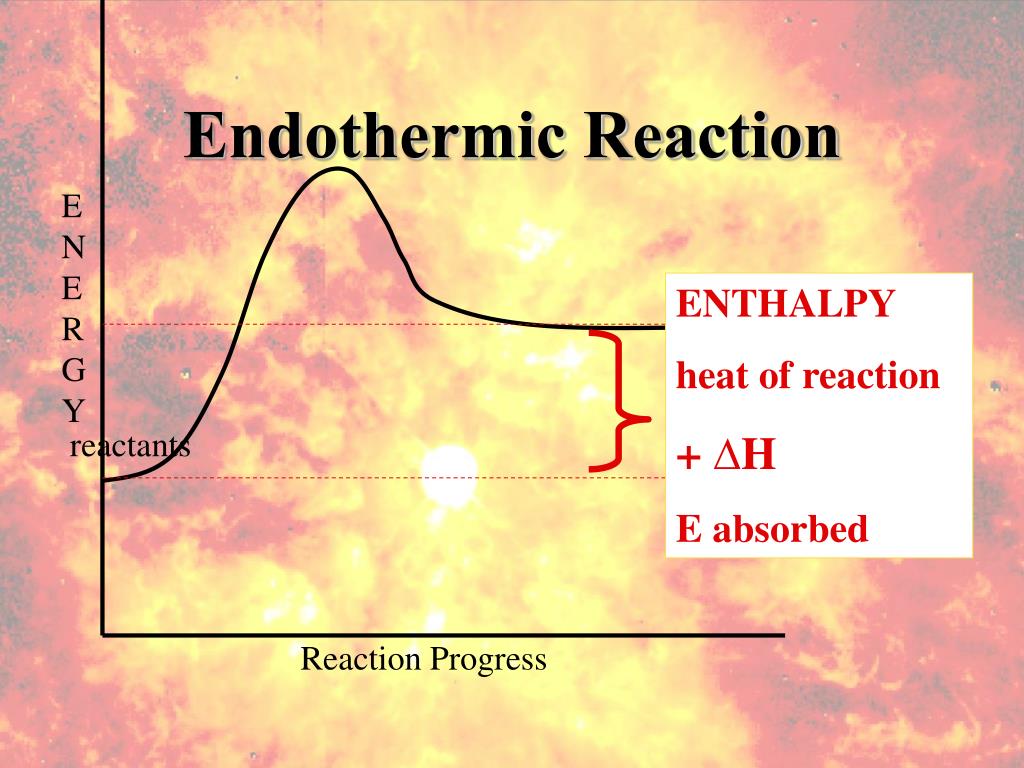
Endothermic Reaction Thermal Energy . Describe how heat is transferred in endothermic and exothermic. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Other chemical reactions release energy in the form of heat, light, or sound. define endothermic and exothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a.
from www.slideserve.com
Other chemical reactions release energy in the form of heat, light, or sound. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. define endothermic and exothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Describe how heat is transferred in endothermic and exothermic. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system.
PPT & Thermodynamics PowerPoint Presentation, free download ID1025110
Endothermic Reaction Thermal Energy Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Describe how heat is transferred in endothermic and exothermic. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. Other chemical reactions release energy in the form of heat, light, or sound. define endothermic and exothermic reactions. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction Thermal Energy Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Describe how heat is transferred in endothermic and exothermic. define endothermic and exothermic reactions. an endothermic reaction is a chemical reaction that. Endothermic Reaction Thermal Energy.
From slidetodoc.com
Chemistry Endothermic and Exothermic Reactions HEAT AND ENERGY Endothermic Reaction Thermal Energy dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. Describe how heat is transferred in endothermic and exothermic. define endothermic and exothermic reactions. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. endothermic reactions are characterized by positive. Endothermic Reaction Thermal Energy.
From schematicorensano5824i.z19.web.core.windows.net
Exothermic And Endothermic Diagrams Endothermic Reaction Thermal Energy Other chemical reactions release energy in the form of heat, light, or sound. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. dissolving ammonium nitrate in water is endothermic because more energy is used to. Endothermic Reaction Thermal Energy.
From stock.adobe.com
Endothermic reactions list with external energy outline collection set. Educational labeled Endothermic Reaction Thermal Energy an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Other chemical reactions release energy in the. Endothermic Reaction Thermal Energy.
From classzonesalicetum.z14.web.core.windows.net
Identify Endothermic And Exothermic Reactions Endothermic Reaction Thermal Energy Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Describe how heat is transferred in endothermic and exothermic. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. define endothermic and exothermic reactions. Other chemical reactions release energy in the. Endothermic Reaction Thermal Energy.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Thermal Energy in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. define endothermic and exothermic reactions. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Describe. Endothermic Reaction Thermal Energy.
From www.slideserve.com
PPT Unit 10 (Chapter 17) Thermodynamics PowerPoint Presentation, free download ID1293884 Endothermic Reaction Thermal Energy dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. define endothermic and exothermic reactions. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Other chemical reactions release energy in the form of heat, light, or sound.. Endothermic Reaction Thermal Energy.
From www.alamy.com
Endothermic reaction (absorbs thermal energy) and exothermic process (releases energy Endothermic Reaction Thermal Energy endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Exothermic reactions may occur spontaneously and result. Endothermic Reaction Thermal Energy.
From pdfslide.net
(PPT) ENERGY AND REACTIONS ENDOTHERMIC AND EXOTHERMIC REACTIONS HEAT OF REACTION BOND ENERGY Endothermic Reaction Thermal Energy Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. define endothermic and exothermic reactions. . Endothermic Reaction Thermal Energy.
From partdiagramdromecanoelq.z19.web.core.windows.net
Exothermic And Endothermic Diagrams Endothermic Reaction Thermal Energy Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Describe how heat is transferred in endothermic and exothermic. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). define endothermic and exothermic reactions. dissolving ammonium nitrate in water is endothermic because. Endothermic Reaction Thermal Energy.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Thermal Energy endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. in endothermic and. Endothermic Reaction Thermal Energy.
From en.ppt-online.org
Thermal Energy, Chemical Energy online presentation Endothermic Reaction Thermal Energy dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. define endothermic and. Endothermic Reaction Thermal Energy.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Thermal Energy in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than. Endothermic Reaction Thermal Energy.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Thermal Energy an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. define endothermic and exothermic reactions. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Other chemical reactions release energy in the form of heat, light, or sound. in endothermic and exothermic reactions, energy can. Endothermic Reaction Thermal Energy.
From kanayatichemistry.blogspot.com
What is an Endothermic Reaction in IGCSE CHEMISTRY ? K Chemistry Endothermic Reaction Thermal Energy define endothermic and exothermic reactions. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. Other chemical reactions release energy in the form of heat, light, or sound. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh).. Endothermic Reaction Thermal Energy.
From app.pandai.org
Thermochemistry Endothermic Reaction Thermal Energy endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic. dissolving ammonium nitrate in water is. Endothermic Reaction Thermal Energy.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Thermal Energy endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Other chemical reactions release. Endothermic Reaction Thermal Energy.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give examples. CBSE Class Notes Endothermic Reaction Thermal Energy endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Describe how heat is transferred in endothermic and exothermic. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction. Endothermic Reaction Thermal Energy.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Thermal Energy an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. define endothermic and exothermic reactions. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Describe how heat is transferred in endothermic and exothermic. dissolving ammonium nitrate in water is endothermic because more energy is. Endothermic Reaction Thermal Energy.
From www.slideserve.com
PPT HEAT AND ENERGY PowerPoint Presentation, free download ID2923328 Endothermic Reaction Thermal Energy Other chemical reactions release energy in the form of heat, light, or sound. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. dissolving ammonium nitrate in water is endothermic because more energy is used to. Endothermic Reaction Thermal Energy.
From wiredatatisse56wr.z22.web.core.windows.net
Endothermic Reaction Energy Profile Diagram Endothermic Reaction Thermal Energy dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. define endothermic and exothermic reactions. Other chemical reactions release energy in the form of heat, light, or sound. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction. Endothermic Reaction Thermal Energy.
From resolutionsforyou.com
Endothermic potential energy diagram Endothermic Reaction Thermal Energy in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Describe how heat is transferred in endothermic and exothermic. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Other chemical reactions release energy in the form of heat, light, or sound.. Endothermic Reaction Thermal Energy.
From www.slideserve.com
PPT HEAT AND ENERGY PowerPoint Presentation, free download ID2923328 Endothermic Reaction Thermal Energy define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). in endothermic and exothermic reactions, energy can be thought of as. Endothermic Reaction Thermal Energy.
From pnghero.com
Photosynthetic Efficiency Exothermic Process Endothermic Reaction Chemical Thermal Energy PNG Endothermic Reaction Thermal Energy dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Other chemical reactions release energy in the form of heat, light, or sound. an endothermic reaction is a. Endothermic Reaction Thermal Energy.
From www.slideserve.com
PPT & Thermodynamics PowerPoint Presentation, free download ID1025110 Endothermic Reaction Thermal Energy endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic. in endothermic and exothermic reactions, energy can be thought of as. Endothermic Reaction Thermal Energy.
From slidetodoc.com
Atoms and Its Parts Bonding Physical Changes Solar Endothermic Reaction Thermal Energy endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Describe how heat is transferred in endothermic and exothermic. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction. Endothermic Reaction Thermal Energy.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID431456 Endothermic Reaction Thermal Energy Describe how heat is transferred in endothermic and exothermic. Other chemical reactions release energy in the form of heat, light, or sound. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. dissolving ammonium nitrate in. Endothermic Reaction Thermal Energy.
From www.expii.com
Energy Diagram — Overview & Parts Expii Endothermic Reaction Thermal Energy in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. endothermic. Endothermic Reaction Thermal Energy.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID3196359 Endothermic Reaction Thermal Energy endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Other chemical reactions release. Endothermic Reaction Thermal Energy.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Thermal Energy define endothermic and exothermic reactions. Other chemical reactions release energy in the form of heat, light, or sound. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. an endothermic. Endothermic Reaction Thermal Energy.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Reaction Thermal Energy an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Describe how heat is transferred in endothermic and exothermic. define endothermic and exothermic reactions. dissolving ammonium nitrate in water is endothermic because more energy is. Endothermic Reaction Thermal Energy.
From slidetodoc.com
Endothermic Exothermic Reactions Enthalpy H Heat Enthalpy Change Endothermic Reaction Thermal Energy define endothermic and exothermic reactions. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in. Endothermic Reaction Thermal Energy.
From www.dreamstime.com
Endothermic Reaction and Exothermic Process Stock Vector Illustration of absorb, physics Endothermic Reaction Thermal Energy dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is. Describe how heat is transferred in endothermic and exothermic. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. an endothermic reaction is a chemical reaction that absorbs. Endothermic Reaction Thermal Energy.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Thermal Energy Other chemical reactions release energy in the form of heat, light, or sound. define endothermic and exothermic reactions. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. dissolving ammonium nitrate in water is endothermic. Endothermic Reaction Thermal Energy.
From general.chemistrysteps.com
What is Enthalpy Chemistry Steps Endothermic Reaction Thermal Energy Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Describe how heat is transferred in endothermic and exothermic. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings.. Endothermic Reaction Thermal Energy.