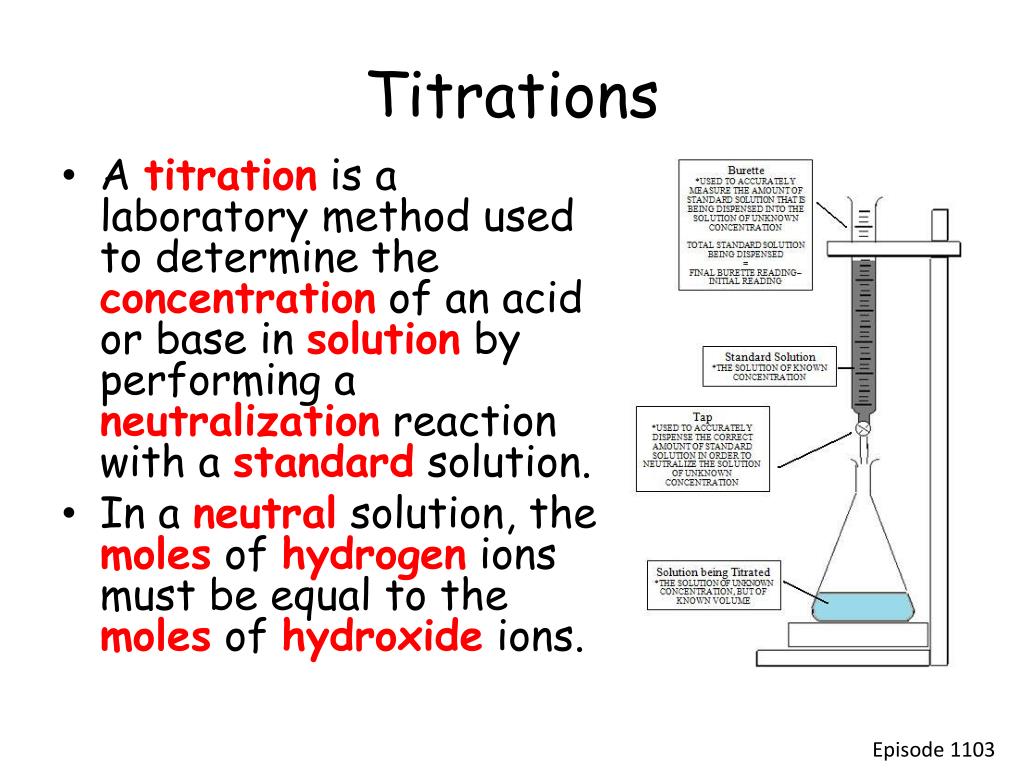
Naoh And Hcl Titration Discussion . In a titration, it is critical to know the exact concentration of the titrant (the. standardizing a sodium hydroxide (naoh) solution. To identify the equivalence point in the titration, we use titration curves and indicators. naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. Includes kit list and safety instructions. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. naoh is a strong alkali and hcl acid is a strong acid respectively. When the acid and base react,. According to the reaction equation. titrate with naoh solution till the first color change. Hydrochloric acid reacts with sodium hydroxide on.
from mungfali.com
titrate with naoh solution till the first color change. When the acid and base react,. In a titration, it is critical to know the exact concentration of the titrant (the. standardizing a sodium hydroxide (naoh) solution. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Hydrochloric acid reacts with sodium hydroxide on. naoh is a strong alkali and hcl acid is a strong acid respectively. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. To identify the equivalence point in the titration, we use titration curves and indicators.
HCl NaOH Titration
Naoh And Hcl Titration Discussion In a titration, it is critical to know the exact concentration of the titrant (the. standardizing a sodium hydroxide (naoh) solution. titrate with naoh solution till the first color change. Hydrochloric acid reacts with sodium hydroxide on. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When the acid and base react,. Includes kit list and safety instructions. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. To identify the equivalence point in the titration, we use titration curves and indicators. In a titration, it is critical to know the exact concentration of the titrant (the. naoh is a strong alkali and hcl acid is a strong acid respectively. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. According to the reaction equation.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Naoh And Hcl Titration Discussion To identify the equivalence point in the titration, we use titration curves and indicators. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. in equation. Naoh And Hcl Titration Discussion.
From dxopcuxtp.blob.core.windows.net
Titration Experiment Hydrochloric Acid And Sodium Hydroxide at Silvia Pearson blog Naoh And Hcl Titration Discussion Hydrochloric acid reacts with sodium hydroxide on. Includes kit list and safety instructions. To identify the equivalence point in the titration, we use titration curves and indicators. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. According to the reaction equation. titrate with naoh. Naoh And Hcl Titration Discussion.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Naoh And Hcl Titration Discussion naoh is a strong alkali and hcl acid is a strong acid respectively. To identify the equivalence point in the titration, we use titration curves and indicators. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Includes kit list and safety instructions. When the acid and base react,. use this. Naoh And Hcl Titration Discussion.
From studymoose.com
Titration with HCl and NaOH Free Essay Example Naoh And Hcl Titration Discussion In a titration, it is critical to know the exact concentration of the titrant (the. When the acid and base react,. naoh is a strong alkali and hcl acid is a strong acid respectively. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. naoh is the excess reagent. Naoh And Hcl Titration Discussion.
From byjus.com
The graph of pH during the titration of NaOH and HCl Naoh And Hcl Titration Discussion naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When the acid and base react,. Includes kit list and safety instructions. the point at which exactly enough titrant. Naoh And Hcl Titration Discussion.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Naoh And Hcl Titration Discussion naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. naoh is a strong alkali and hcl acid is a strong acid respectively. According to the reaction equation. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). standardizing a. Naoh And Hcl Titration Discussion.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Naoh And Hcl Titration Discussion To identify the equivalence point in the titration, we use titration curves and indicators. Hydrochloric acid reacts with sodium hydroxide on. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. According to the. Naoh And Hcl Titration Discussion.
From dxopcuxtp.blob.core.windows.net
Titration Experiment Hydrochloric Acid And Sodium Hydroxide at Silvia Pearson blog Naoh And Hcl Titration Discussion in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). titrate with naoh solution till the first color change. standardizing a sodium hydroxide (naoh) solution. According to the reaction equation. naoh is a strong alkali and hcl acid is a strong acid respectively. Hydrochloric acid reacts with sodium hydroxide on.. Naoh And Hcl Titration Discussion.
From mungfali.com
HCl NaOH Titration Naoh And Hcl Titration Discussion According to the reaction equation. When the acid and base react,. Includes kit list and safety instructions. To identify the equivalence point in the titration, we use titration curves and indicators. In a titration, it is critical to know the exact concentration of the titrant (the. naoh is a strong alkali and hcl acid is a strong acid respectively.. Naoh And Hcl Titration Discussion.
From www.youtube.com
NaOH vs HCl Titration using Phenolphthalein Class 11 Chemistry Lab Practical Practical Copy Naoh And Hcl Titration Discussion standardizing a sodium hydroxide (naoh) solution. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Includes kit list and safety instructions. According to the reaction equation. To identify the equivalence point in the titration, we use titration curves and indicators. the point at which exactly enough titrant (naoh) has been. Naoh And Hcl Titration Discussion.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Naoh And Hcl Titration Discussion titrate with naoh solution till the first color change. To identify the equivalence point in the titration, we use titration curves and indicators. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. naoh is the excess reagent and hcl is the limiting. Naoh And Hcl Titration Discussion.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Naoh And Hcl Titration Discussion In a titration, it is critical to know the exact concentration of the titrant (the. naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. According to the reaction equation. To identify the equivalence point in the titration, we use titration curves and indicators. When the acid and base. Naoh And Hcl Titration Discussion.
From studylib.net
Titration of HCl with NaOH Naoh And Hcl Titration Discussion To identify the equivalence point in the titration, we use titration curves and indicators. Includes kit list and safety instructions. According to the reaction equation. In a titration, it is critical to know the exact concentration of the titrant (the. Hydrochloric acid reacts with sodium hydroxide on. the point at which exactly enough titrant (naoh) has been added to. Naoh And Hcl Titration Discussion.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Naoh And Hcl Titration Discussion When the acid and base react,. Hydrochloric acid reacts with sodium hydroxide on. In a titration, it is critical to know the exact concentration of the titrant (the. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. naoh is a strong alkali and hcl. Naoh And Hcl Titration Discussion.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Naoh And Hcl Titration Discussion In a titration, it is critical to know the exact concentration of the titrant (the. Hydrochloric acid reacts with sodium hydroxide on. According to the reaction equation. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). the point at which exactly enough titrant (naoh) has been added to react with all. Naoh And Hcl Titration Discussion.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Education Naoh And Hcl Titration Discussion In a titration, it is critical to know the exact concentration of the titrant (the. According to the reaction equation. Includes kit list and safety instructions. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. Hydrochloric acid reacts with sodium hydroxide on. use this. Naoh And Hcl Titration Discussion.
From mariela-kcarroll.blogspot.com
Titration Curve of Hcl and Naoh Naoh And Hcl Titration Discussion To identify the equivalence point in the titration, we use titration curves and indicators. naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. standardizing a sodium hydroxide (naoh) solution. When the acid and base react,. in equation 1, the acid is hcl (hydrochloric acid) and the. Naoh And Hcl Titration Discussion.
From www.chegg.com
Solved Method Titration of sodium hydroxide and hydrochloric Naoh And Hcl Titration Discussion use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on. When the acid and base react,. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. in equation 1,. Naoh And Hcl Titration Discussion.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base (NaOH) YouTube Naoh And Hcl Titration Discussion To identify the equivalence point in the titration, we use titration curves and indicators. naoh is a strong alkali and hcl acid is a strong acid respectively. In a titration, it is critical to know the exact concentration of the titrant (the. According to the reaction equation. standardizing a sodium hydroxide (naoh) solution. titrate with naoh solution. Naoh And Hcl Titration Discussion.
From mungfali.com
HCl NaOH Titration Naoh And Hcl Titration Discussion use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl). Naoh And Hcl Titration Discussion.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Naoh And Hcl Titration Discussion in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). titrate with naoh solution till the first color change. Hydrochloric acid reacts with sodium hydroxide on. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. naoh is a strong alkali and. Naoh And Hcl Titration Discussion.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Naoh And Hcl Titration Discussion Includes kit list and safety instructions. When the acid and base react,. Hydrochloric acid reacts with sodium hydroxide on. According to the reaction equation. titrate with naoh solution till the first color change. standardizing a sodium hydroxide (naoh) solution. naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl,. Naoh And Hcl Titration Discussion.
From www.studypool.com
SOLUTION Titration of naoh with hcl Studypool Naoh And Hcl Titration Discussion naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. According to the reaction equation. When the acid and base react,. To identify the equivalence point in the titration, we use titration curves and indicators. Hydrochloric acid reacts with sodium hydroxide on. the point at which exactly enough. Naoh And Hcl Titration Discussion.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Purpose The purpose of Naoh And Hcl Titration Discussion the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. naoh is a strong alkali and hcl acid is a strong acid respectively. naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. . Naoh And Hcl Titration Discussion.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Naoh And Hcl Titration Discussion To identify the equivalence point in the titration, we use titration curves and indicators. Includes kit list and safety instructions. Hydrochloric acid reacts with sodium hydroxide on. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. naoh is the excess reagent and hcl is the limiting reagent (once you. Naoh And Hcl Titration Discussion.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Naoh And Hcl Titration Discussion standardizing a sodium hydroxide (naoh) solution. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When the acid and base react,. naoh is the excess reagent and hcl is the limiting. Naoh And Hcl Titration Discussion.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Naoh And Hcl Titration Discussion titrate with naoh solution till the first color change. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. standardizing a sodium hydroxide (naoh) solution. When the acid and base react,. Includes kit list and safety instructions. To identify the equivalence point in the titration, we use titration curves. Naoh And Hcl Titration Discussion.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Chemistry Laboratory Naoh And Hcl Titration Discussion use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. titrate with naoh solution till the first color change. naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. In a titration, it is critical to know the exact. Naoh And Hcl Titration Discussion.
From mariela-kcarroll.blogspot.com
Titration Curve of Hcl and Naoh Naoh And Hcl Titration Discussion titrate with naoh solution till the first color change. In a titration, it is critical to know the exact concentration of the titrant (the. According to the reaction equation. Hydrochloric acid reacts with sodium hydroxide on. To identify the equivalence point in the titration, we use titration curves and indicators. Includes kit list and safety instructions. When the acid. Naoh And Hcl Titration Discussion.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Naoh And Hcl Titration Discussion In a titration, it is critical to know the exact concentration of the titrant (the. naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. naoh is a strong alkali and hcl acid is a strong acid respectively. Includes kit list and safety instructions. titrate with naoh. Naoh And Hcl Titration Discussion.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Naoh And Hcl Titration Discussion naoh is a strong alkali and hcl acid is a strong acid respectively. standardizing a sodium hydroxide (naoh) solution. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any. Naoh And Hcl Titration Discussion.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Naoh And Hcl Titration Discussion Hydrochloric acid reacts with sodium hydroxide on. naoh is a strong alkali and hcl acid is a strong acid respectively. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. According to the reaction equation. Includes kit list and safety instructions. To identify the equivalence. Naoh And Hcl Titration Discussion.
From mungfali.com
HCl NaOH Titration Naoh And Hcl Titration Discussion naoh is a strong alkali and hcl acid is a strong acid respectively. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. the point at which exactly enough titrant (naoh) has been added to react with all of the analyte (hcl) is called the equivalence. naoh is. Naoh And Hcl Titration Discussion.
From www.youtube.com
Titration HCl and NaOH methyl orange YouTube Naoh And Hcl Titration Discussion use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. To identify the equivalence point in the titration, we use titration curves and indicators. According to the reaction equation. naoh is a strong alkali and hcl acid is a strong acid respectively. standardizing a sodium hydroxide (naoh) solution. . Naoh And Hcl Titration Discussion.
From mungfali.com
HCl NaOH Titration Naoh And Hcl Titration Discussion According to the reaction equation. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. naoh is the excess reagent and hcl is the limiting reagent (once you have neutralized all the hcl, any more. the point at which exactly enough titrant (naoh) has been added to react with. Naoh And Hcl Titration Discussion.