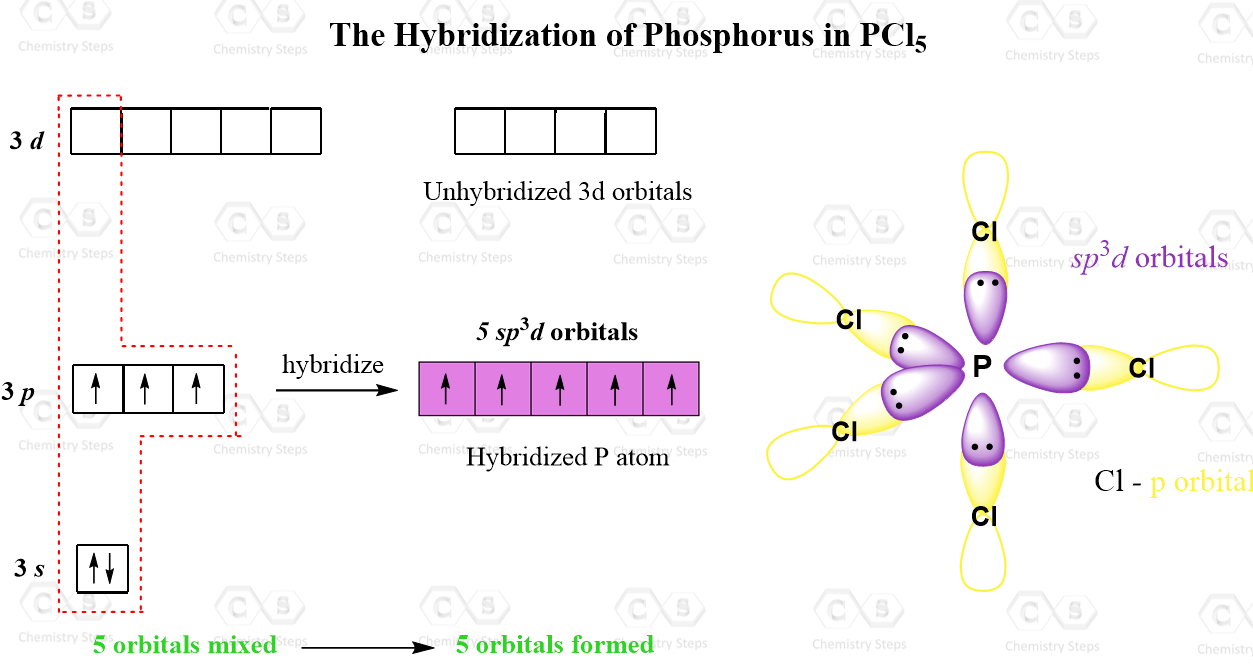
Hybridization Used By . hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. In it, the 2s orbital and one of the 2p orbitals. As you know, every atom has electron orbitals surrounding it. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. Sp hybridization can explain the linear structure in molecules.
from general.chemistrysteps.com
Sp hybridization can explain the linear structure in molecules. hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. As you know, every atom has electron orbitals surrounding it. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. In it, the 2s orbital and one of the 2p orbitals. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not.
Hybridization of Atomic Orbitals Chemistry Steps
Hybridization Used By As you know, every atom has electron orbitals surrounding it. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. As you know, every atom has electron orbitals surrounding it. Sp hybridization can explain the linear structure in molecules. In it, the 2s orbital and one of the 2p orbitals. hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not.
From www.leadingbiology.com
In Situ Hybridization_Immunology Services_Custom Services_Leading Hybridization Used By hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an. Hybridization Used By.
From ar.inspiredpencil.com
Hybridization Chart Hybridization Used By Sp hybridization can explain the linear structure in molecules. In it, the 2s orbital and one of the 2p orbitals. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of. Hybridization Used By.
From ar.inspiredpencil.com
Hybridization Chart Shape Hybridization Used By Sp hybridization can explain the linear structure in molecules. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. As you know, every atom has electron orbitals surrounding it. hybridization, in chemistry, is defined as the concept of mixing two atomic. Hybridization Used By.
From www.slideserve.com
PPT Modern Agriculture PowerPoint Presentation, free download ID Hybridization Used By the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. Sp hybridization can explain the linear structure in molecules. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. In it, the 2s orbital and one. Hybridization Used By.
From byjus.com
Hybridization of Carbon Molecular Geometry and Bond Angles Hybridization Used By Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. hybridization is the concept of mixing or combining of. Hybridization Used By.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Hybridization Used By hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom. Hybridization Used By.
From www.chemistrylibrary.org
Types of Hybridization Hybridization Used By hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. Sp hybridization can explain the linear. Hybridization Used By.
From cstuart7.blogspot.com
Cole's AP Chem Blog October 2013 Hybridization Used By In it, the 2s orbital and one of the 2p orbitals. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type. Hybridization Used By.
From avantecnica.qualitypoolsboulder.com
Hybridization Definition, Types, Rules, Examples Hybridization Used By the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. Sp hybridization can explain the linear structure in molecules. As you know, every atom has electron orbitals surrounding it. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can. Hybridization Used By.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Used By hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. As you know, every atom has electron orbitals surrounding it. Sp hybridization can explain the linear structure. Hybridization Used By.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Used By hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. In it, the 2s orbital and one of the 2p orbitals. hybridization is the concept. Hybridization Used By.
From askfilo.com
Explain in detail the hybridization of following Filo Hybridization Used By Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. hybridization, in chemistry, is defined as the concept of mixing. Hybridization Used By.
From chemistrytalk.org
Hybridization and Hybrid Orbitals ChemTalk Hybridization Used By Sp hybridization can explain the linear structure in molecules. As you know, every atom has electron orbitals surrounding it. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of. Hybridization Used By.
From thechemistrynotes.com
Hybridization Definition, Features, Types Hybridization Used By the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. hybridization is defined as the phenomenon of mixing up. Hybridization Used By.
From mungfali.com
Hybridization Periodic Table Hybridization Used By hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. In it, the 2s orbital and one of the 2p orbitals. Sp hybridization can explain the linear structure in molecules. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of. Hybridization Used By.
From eduinput.com
Hybridization, definition, types and significance Hybridization Used By As you know, every atom has electron orbitals surrounding it. hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. . Hybridization Used By.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Used By Sp hybridization can explain the linear structure in molecules. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. the localized. Hybridization Used By.
From www.geeksforgeeks.org
Hybridization Definition, Types, Rules, Examples Hybridization Used By As you know, every atom has electron orbitals surrounding it. In it, the 2s orbital and one of the 2p orbitals. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom. Hybridization Used By.
From www.labtestsguide.com
FISH (Fluorescence In Situ Hybridization) Purpose, procedure Hybridization Used By hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. Sp hybridization can explain the linear structure in molecules. As you know, every atom has electron orbitals surrounding it. In it, the 2s orbital and one of the 2p orbitals. hybridization, in chemistry, is defined as the concept. Hybridization Used By.
From www.adda247.com
What is Hybridization? sp3, sp2, Examples and Formula Hybridization Used By hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. Sp hybridization can explain the linear structure in molecules. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. Simply put, hybridization is the way that. Hybridization Used By.
From www.genome.gov
Hybridization Hybridization Used By hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. In it, the 2s orbital and one of the 2p orbitals. Sp hybridization can explain the linear structure in molecules. As you know, every atom has electron orbitals surrounding it. hybridization, in chemistry, is defined as the concept. Hybridization Used By.
From geneticeducation.co.in
What is DNA hybridization and How does it occur? Education Hybridization Used By In it, the 2s orbital and one of the 2p orbitals. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. As you know, every atom has electron orbitals surrounding it. hybridization is defined as the phenomenon of mixing up (or. Hybridization Used By.
From www.researchgate.net
Basic steps of fluorescent in situ hybridization technique DNA Hybridization Used By hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. Simply put, hybridization is the way that distinct atomic orbitals combine together. Hybridization Used By.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps Hybridization Used By Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. In it, the 2s orbital and one of the 2p orbitals. As you know, every atom has electron orbitals surrounding it. the localized valence bond theory uses a process called hybridization,. Hybridization Used By.
From ar.inspiredpencil.com
Hybridization Orbitals Chart Hybridization Used By the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise. Hybridization Used By.
From chemwiki.ucdavis.edu
10.3 Hybridization of Atomic Orbitals Chemwiki Hybridization Used By In it, the 2s orbital and one of the 2p orbitals. Sp hybridization can explain the linear structure in molecules. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to. Hybridization Used By.
From gamesmartz.com
Hybridization Definition & Image GameSmartz Hybridization Used By hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. hybridization is defined as the phenomenon of mixing up (or. Hybridization Used By.
From classnotes.org.in
Hybridisation Chemical Bonding and Molecular Structure, Chemistry Hybridization Used By hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. As you know, every atom has electron orbitals surrounding it. hybridization is the concept of. Hybridization Used By.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Hybridization Used By As you know, every atom has electron orbitals surrounding it. hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. Sp hybridization can explain the linear structure in molecules. In it, the 2s orbital and one of the 2p orbitals. hybridization, in chemistry, is defined as the concept. Hybridization Used By.
From chemistnotes.com
Hybridization Definition, types and examples Chemistry Notes Hybridization Used By hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. In it, the 2s orbital and one of the 2p orbitals. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much. Hybridization Used By.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Used By Sp hybridization can explain the linear structure in molecules. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. Simply put, hybridization is. Hybridization Used By.
From www.youtube.com
HYBRIDIZATION part 3 YouTube Hybridization Used By Sp hybridization can explain the linear structure in molecules. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. Simply put, hybridization. Hybridization Used By.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Used By the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. In it, the 2s orbital and one of the 2p orbitals. Simply put, hybridization is the way. Hybridization Used By.
From www.genome.gov
Hybridization Hybridization Used By As you know, every atom has electron orbitals surrounding it. Sp hybridization can explain the linear structure in molecules. In it, the 2s orbital and one of the 2p orbitals. hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. hybridization is defined as the phenomenon of mixing. Hybridization Used By.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry Hybridization Used By hybridization is the concept of mixing or combining of two atomic orbitals to create a new type of hybridized orbitals. the localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but not. Sp hybridization can explain the linear structure in molecules. As you know, every atom has electron orbitals. Hybridization Used By.