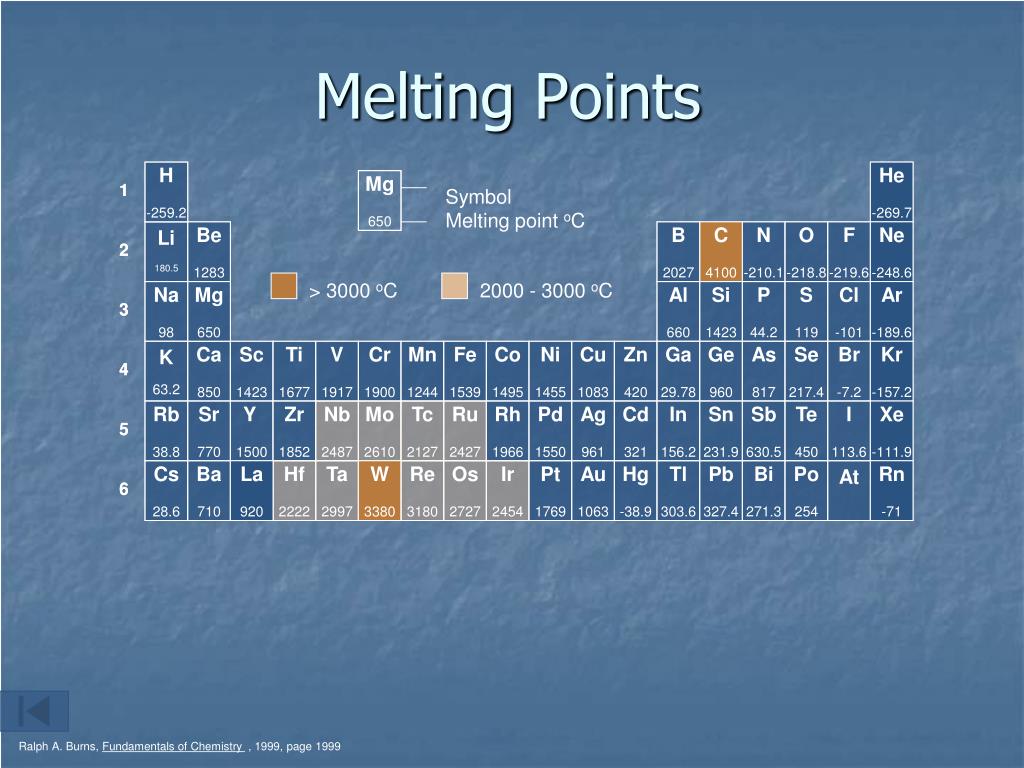
Why Is Melting Point A Periodic Property . Learn how the melting points of the elements across period 3 vary due to their bonding and structure. The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. 119 rows the melting point of an element is the energy required to break the bonds between the atoms. This is because the atoms get bigger and the forces of attraction between them get. For the elements, color does not vary. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. Learn how melting point is used to identify compounds and elements, and how it. The melting point of the nitrogen. Generally, the stronger the bond between. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist.
from www.slideserve.com
Generally, the stronger the bond between. The melting point of the nitrogen. The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. This is because the atoms get bigger and the forces of attraction between them get. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Learn how melting point is used to identify compounds and elements, and how it. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. For the elements, color does not vary. Learn how the melting points of the elements across period 3 vary due to their bonding and structure. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point.
PPT Periodic Table PowerPoint Presentation, free download ID3389286
Why Is Melting Point A Periodic Property The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. This is because the atoms get bigger and the forces of attraction between them get. Generally, the stronger the bond between. Learn how the melting points of the elements across period 3 vary due to their bonding and structure. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. The melting point of the nitrogen. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. For the elements, color does not vary. 119 rows the melting point of an element is the energy required to break the bonds between the atoms. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. Learn how melting point is used to identify compounds and elements, and how it.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint Why Is Melting Point A Periodic Property The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. The melting point of the nitrogen. Learn how melting point is used to identify compounds and elements, and how it. 119 rows the melting point of an element is the energy required to break the bonds between the atoms. Physical properties. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint Why Is Melting Point A Periodic Property The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. For the elements, color does not vary. This is because the atoms get bigger and the forces of attraction between them get. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. Melting point. Why Is Melting Point A Periodic Property.
From www.youtube.com
PROPERTY OF D BLOCK ELEMENTS (MELTING POINT AND BOILING POINT) YouTube Why Is Melting Point A Periodic Property The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. For. Why Is Melting Point A Periodic Property.
From www.worksheetsplanet.com
What is Melting Point Definition of Melting Point Why Is Melting Point A Periodic Property Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. 119 rows the melting point of an element is the energy required to break the bonds between the atoms. Generally, the stronger the bond between. The melting point of the group 1 elements decreases as you go down the group, from lithium to. Why Is Melting Point A Periodic Property.
From www.youtube.com
How to Remember Melting Point and Boiling Points of Full Periodic Table Why Is Melting Point A Periodic Property The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. Melting point is the temperature at which a solid and a liquid of a pure substance can. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Variation in Physical Properties of sBlock elements PowerPoint Why Is Melting Point A Periodic Property Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. Learn. Why Is Melting Point A Periodic Property.
From www.tes.com
Periodic Trends Metallic Bonding and Melting Points Teaching Resources Why Is Melting Point A Periodic Property Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. Learn how the melting points of the elements across period 3 vary due to their bonding and structure. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. For the elements, color does not vary.. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Periodic Properties of Elements in the Periodic Table PowerPoint Why Is Melting Point A Periodic Property Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. For the elements, color does not vary. The melting point of the nitrogen. 119 rows the melting point of an element is the energy required to break the bonds between the atoms. Learn how the melting points of the elements across period 3. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Topic 3 Periodicity PowerPoint Presentation, free download ID Why Is Melting Point A Periodic Property Learn how the melting points of the elements across period 3 vary due to their bonding and structure. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. For the elements, color does not vary. Generally, the stronger the bond between. The melting point of the group 1 elements decreases as you go. Why Is Melting Point A Periodic Property.
From revisezone.com
Periodicity Notes Period 3 Revise Zone Why Is Melting Point A Periodic Property Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. Learn how the melting points of the elements across period 3 vary due to their bonding and structure. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. The. Why Is Melting Point A Periodic Property.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Why Is Melting Point A Periodic Property Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. 119 rows the melting point of an element is the energy required to break the bonds between the. Why Is Melting Point A Periodic Property.
From sciencenotes.org
Melting Point Definition and List Why Is Melting Point A Periodic Property This is because the atoms get bigger and the forces of attraction between them get. Generally, the stronger the bond between. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. Learn how melting point is used to identify compounds and elements, and how it. For the elements, color does not vary.. Why Is Melting Point A Periodic Property.
From www.slideshare.net
Periodic Properties Of Elements In The Periodic Table Why Is Melting Point A Periodic Property The melting point of the nitrogen. Learn how the melting points of the elements across period 3 vary due to their bonding and structure. This is because the atoms get bigger and the forces of attraction between them get. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance. Why Is Melting Point A Periodic Property.
From winter.group.shef.ac.uk
Elements Periodic Table » Periodicity » Melting point » Sized balls Why Is Melting Point A Periodic Property Learn how melting point is used to identify compounds and elements, and how it. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. This is because. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Melting Point and Boiling Point PowerPoint Presentation, free Why Is Melting Point A Periodic Property Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. Learn how the melting points of the elements across period 3 vary due to their bonding and structure. 119 rows the melting point of an element is the energy required to break the bonds between the atoms. Melting point is the temperature at. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT 103 PowerPoint Presentation, free download ID6601161 Why Is Melting Point A Periodic Property Generally, the stronger the bond between. The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. This is because the atoms get bigger and the forces of attraction between them get. Learn how melting point is used to identify compounds and elements, and how it. 119 rows the melting point of. Why Is Melting Point A Periodic Property.
From studylib.net
Chapter 7 Periodic Properties of the Elements Why Is Melting Point A Periodic Property The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. Learn how the melting points of the elements across period 3 vary due to their bonding and structure.. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint Why Is Melting Point A Periodic Property Learn how the melting points of the elements across period 3 vary due to their bonding and structure. Learn how melting point is used to identify compounds and elements, and how it. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. The melting points is the amount of energy required to break. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint Why Is Melting Point A Periodic Property The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. The melting point of the nitrogen.. Why Is Melting Point A Periodic Property.
From fity.club
Melting Points And Boiling Points Why Is Melting Point A Periodic Property Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Generally,. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Melting Point PowerPoint Presentation, free download ID3063314 Why Is Melting Point A Periodic Property Learn how melting point is used to identify compounds and elements, and how it. For the elements, color does not vary. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. Generally, the. Why Is Melting Point A Periodic Property.
From pubchem.ncbi.nlm.nih.gov
Melting Point Periodic Table of Elements PubChem Why Is Melting Point A Periodic Property The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. This is because the atoms get bigger and the forces of attraction between them get. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. The melting point of the nitrogen. For the. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Physical Properties of Matter PowerPoint Presentation, free Why Is Melting Point A Periodic Property The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. Learn how melting point is used to identify compounds and elements, and how it. The melting point of the nitrogen. Learn how the melting points of the elements across period 3 vary due to their bonding and structure. For the elements,. Why Is Melting Point A Periodic Property.
From msestudent.com
17 Metals With the Highest Melting Points (and Why) Materials Science Why Is Melting Point A Periodic Property The melting point of the group 1 elements decreases as you go down the group, from lithium to caesium. For the elements, color does not vary. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Learn how the melting points of the elements across period. Why Is Melting Point A Periodic Property.
From giorwqmki.blob.core.windows.net
What Is A Melting Point Temperature at Patricia Nicholson blog Why Is Melting Point A Periodic Property Generally, the stronger the bond between. Learn how melting point is used to identify compounds and elements, and how it. For the elements, color does not vary. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Melting point is the temperature at which a solid. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID3389286 Why Is Melting Point A Periodic Property The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Learn how the melting points of the elements across period 3 vary due to their bonding and structure. For the elements, color does not vary. The melting point of the group 1 elements decreases as you. Why Is Melting Point A Periodic Property.
From winter.group.shef.ac.uk
Elements Periodic Table » Periodicity » Melting point » Sized balls Why Is Melting Point A Periodic Property 119 rows the melting point of an element is the energy required to break the bonds between the atoms. Learn how the melting points of the elements across period 3 vary due to their bonding and structure. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. Learn how melting point is used. Why Is Melting Point A Periodic Property.
From ar.inspiredpencil.com
Periodic Table Melting Point Why Is Melting Point A Periodic Property The melting point of the nitrogen. Generally, the stronger the bond between. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. This is because the atoms get bigger. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID5757958 Why Is Melting Point A Periodic Property Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Generally,. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Melting Points of Alkali Metals PowerPoint Presentation, free Why Is Melting Point A Periodic Property 119 rows the melting point of an element is the energy required to break the bonds between the atoms. This is because the atoms get bigger and the forces of attraction between them get. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. Generally, the stronger the bond between. The melting point. Why Is Melting Point A Periodic Property.
From msestudent.com
17 Metals With the Highest Melting Points (and Why) Materials Science Why Is Melting Point A Periodic Property The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. Generally, the stronger the bond between. 119 rows the melting point of an element is the energy required. Why Is Melting Point A Periodic Property.
From sciencenotes.org
Melting Point Definition and List Why Is Melting Point A Periodic Property The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. For the elements, color does not vary. Melting point is the temperature at which a solid and a liquid of a pure substance can coexist. The melting point of the nitrogen. 119 rows the melting point. Why Is Melting Point A Periodic Property.
From www.researchgate.net
Melting and boiling points for the elements of the periodic table (data Why Is Melting Point A Periodic Property Learn how melting point is used to identify compounds and elements, and how it. Generally, the stronger the bond between. The melting points is the amount of energy required to break a bond(s) to change the solid phase of a substance to a liquid. The melting point of the nitrogen. Melting point is the temperature at which a solid and. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint Why Is Melting Point A Periodic Property 119 rows the melting point of an element is the energy required to break the bonds between the atoms. Generally, the stronger the bond between. The melting point of the nitrogen. Learn how melting point is used to identify compounds and elements, and how it. Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and. Why Is Melting Point A Periodic Property.
From www.slideserve.com
PPT Physical and Chemical Properties 9/16/2014 PowerPoint Why Is Melting Point A Periodic Property For the elements, color does not vary. Generally, the stronger the bond between. Learn how the melting points of the elements across period 3 vary due to their bonding and structure. Learn how melting point is used to identify compounds and elements, and how it. Melting point is the temperature at which a solid and a liquid of a pure. Why Is Melting Point A Periodic Property.