What Is The Number Of Electrons Shared In N2 . The total number of electrons present in the valence shell is 5 * 2 = 10e. These are then paired up in an opposite spin (pauli exclusion principle). Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. Therefore, nitrogen molecule is formed by. Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. Total 6 electrons are shared in n₂. Nitrogen is belong to group va. In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x 2. It have 5 electron in its outer most shell. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule).
from utedzz.blogspot.com
Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. Therefore, nitrogen molecule is formed by. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). It have 5 electron in its outer most shell. Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. Total 6 electrons are shared in n₂. Nitrogen is belong to group va. These are then paired up in an opposite spin (pauli exclusion principle).
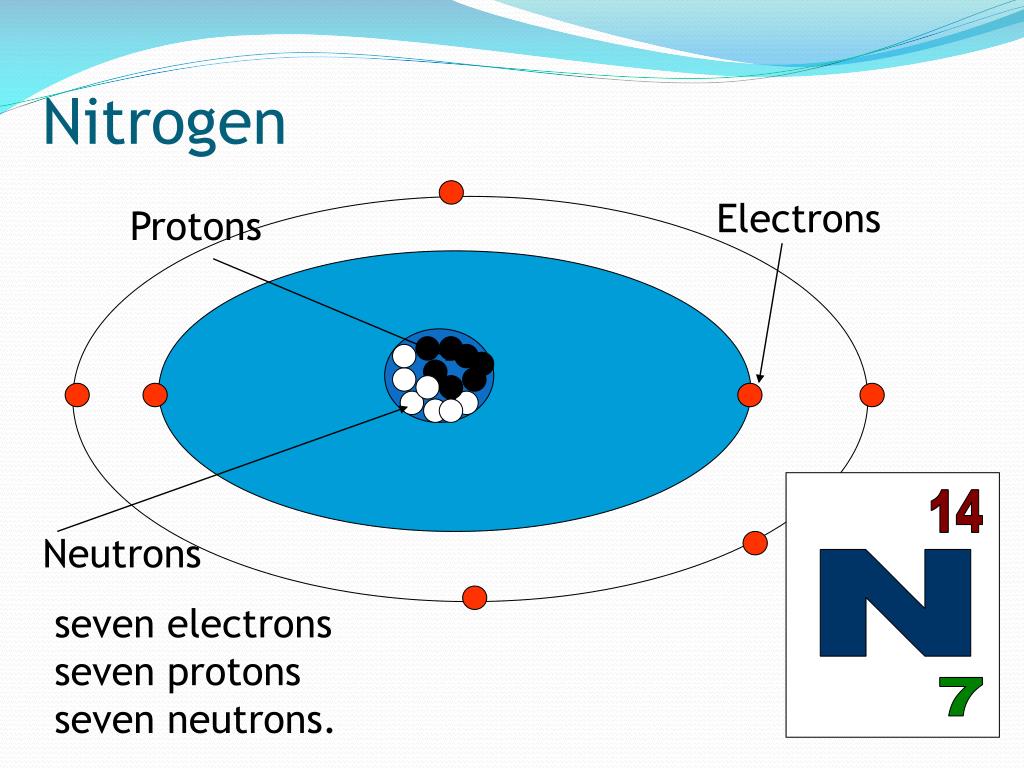
Nitrogen Periodic Table Protons Neutrons Electrons Periodic Table
What Is The Number Of Electrons Shared In N2 These are then paired up in an opposite spin (pauli exclusion principle). Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. The total number of electrons present in the valence shell is 5 * 2 = 10e. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: These are then paired up in an opposite spin (pauli exclusion principle). Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. It have 5 electron in its outer most shell. Total 6 electrons are shared in n₂. In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x 2. Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. Therefore, nitrogen molecule is formed by. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). Nitrogen is belong to group va.
From www.doubtnut.com
The number of electrons shared by each N atom in N2 is What Is The Number Of Electrons Shared In N2 Therefore, nitrogen molecule is formed by. Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: In this problem, we need to find the number of electrons shared between the atoms in a molecule of. What Is The Number Of Electrons Shared In N2.
From howtodiscuss.com
Lewis Structure for N2 How To Discuss What Is The Number Of Electrons Shared In N2 Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. It have 5 electron in its outer most shell. These are then paired up in an opposite spin (pauli exclusion principle).. What Is The Number Of Electrons Shared In N2.
From www.nagwa.com
Question Video Writing the Electronic Configuration of the Nitrogen What Is The Number Of Electrons Shared In N2 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). These are then paired up in an opposite spin (pauli exclusion principle). Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. Therefore, nitrogen molecule. What Is The Number Of Electrons Shared In N2.
From ask4essay.com
How many valence electrons does nitrogen have? Ask4Essay What Is The Number Of Electrons Shared In N2 Total 6 electrons are shared in n₂. It have 5 electron in its outer most shell. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). These are then paired up in an opposite spin (pauli exclusion principle). Study with quizlet and memorize flashcards containing terms like. What Is The Number Of Electrons Shared In N2.
From www.goodscience.com.au
Ionic and Covalent Compounds Good Science What Is The Number Of Electrons Shared In N2 The total number of electrons present in the valence shell is 5 * 2 = 10e. Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. Therefore, nitrogen molecule is formed by. It have 5 electron in its outer most shell. In this problem, we need to find the number of. What Is The Number Of Electrons Shared In N2.
From valenceelectrons.com
How to Write the Electron Configuration for Nitrogen and N3 What Is The Number Of Electrons Shared In N2 Total 6 electrons are shared in n₂. Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). The total number of electrons present in the valence shell is 5 *. What Is The Number Of Electrons Shared In N2.
From phootoscelebrities.com
Is N2 Polar Or Nonpolar? What Is The Number Of Electrons Shared In N2 Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). Nitrogen molecule is diatomic and contains a triple bond between two n atoms: In this problem, we need to find. What Is The Number Of Electrons Shared In N2.
From www.youtube.com
How to write the Electronic Configuration of Nitrogen Chemical What Is The Number Of Electrons Shared In N2 Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2. What Is The Number Of Electrons Shared In N2.
From valenceelectrons.com
What are valence electrons and why its important? What Is The Number Of Electrons Shared In N2 Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. In this problem, we need to find the number of electrons shared between the atoms in a molecule of. What Is The Number Of Electrons Shared In N2.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 What Is The Number Of Electrons Shared In N2 The total number of electrons present in the valence shell is 5 * 2 = 10e. Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: Therefore, nitrogen molecule is formed by. 2 out of. What Is The Number Of Electrons Shared In N2.
From www.britannica.com
nitrogen Definition, Symbol, Uses, Properties, Atomic Number, & Facts What Is The Number Of Electrons Shared In N2 In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x 2. Nitrogen is belong to group va. Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. Each nitrogen atom has five valence electrons shared. What Is The Number Of Electrons Shared In N2.
From www.doubtnut.com
Describe the formation of nitrogen molecule. What Is The Number Of Electrons Shared In N2 Nitrogen molecule is diatomic and contains a triple bond between two n atoms: The total number of electrons present in the valence shell is 5 * 2 = 10e. Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. These are then paired up in an opposite spin (pauli exclusion principle).. What Is The Number Of Electrons Shared In N2.
From material-properties.org
Nitrogen Periodic Table and Atomic Properties What Is The Number Of Electrons Shared In N2 These are then paired up in an opposite spin (pauli exclusion principle). Total 6 electrons are shared in n₂. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). Therefore, nitrogen molecule is formed by. Nitrogen is belong to group va. In this problem, we need to. What Is The Number Of Electrons Shared In N2.
From periodictable.me
Nitrogen Electron Configuration (N) with Orbital Diagram What Is The Number Of Electrons Shared In N2 In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x 2. The total number of electrons present in the valence shell is 5 * 2 = 10e. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: Study with quizlet and. What Is The Number Of Electrons Shared In N2.
From www.slideserve.com
PPT Electron configurations tells us in which orbitals the electrons What Is The Number Of Electrons Shared In N2 Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. The total number of electrons present in the valence shell is 5 * 2 = 10e. Nitrogen is belong to group va. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: In this problem, we need to find the. What Is The Number Of Electrons Shared In N2.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Nitrogen (N What Is The Number Of Electrons Shared In N2 The total number of electrons present in the valence shell is 5 * 2 = 10e. Total 6 electrons are shared in n₂. In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x 2. Study with quizlet and memorize flashcards containing terms like which. What Is The Number Of Electrons Shared In N2.
From ar.inspiredpencil.com
Nitrogen Symbol Atomic Number Atomic Mass What Is The Number Of Electrons Shared In N2 Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. Total 6 electrons are shared in n₂. These are then paired up in an opposite spin (pauli exclusion principle). Nitrogen molecule is diatomic and contains a triple bond between two n atoms: Thus, 10 valence electrons need to be arranged in. What Is The Number Of Electrons Shared In N2.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic What Is The Number Of Electrons Shared In N2 Nitrogen molecule is diatomic and contains a triple bond between two n atoms: The total number of electrons present in the valence shell is 5 * 2 = 10e. Therefore, nitrogen molecule is formed by. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). Thus, 10. What Is The Number Of Electrons Shared In N2.
From manuallistcantabank.z21.web.core.windows.net
Lewis Diagram For N2 What Is The Number Of Electrons Shared In N2 Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: Nitrogen is belong to group va. The total number of electrons present in the valence shell is 5 * 2 = 10e. It have 5 electron in its outer most. What Is The Number Of Electrons Shared In N2.
From galvinconanstuart.blogspot.com
Lewis Dot Diagram For N2 General Wiring Diagram What Is The Number Of Electrons Shared In N2 In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x 2. Nitrogen is belong to group va. Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. It have 5 electron in its outer most. What Is The Number Of Electrons Shared In N2.
From www.slideserve.com
PPT Chemical Bonds The Formation of Compounds From Atoms PowerPoint What Is The Number Of Electrons Shared In N2 Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). Therefore, nitrogen molecule is formed by. Each nitrogen atom has five valence electrons shared with another nitrogen atom in order. What Is The Number Of Electrons Shared In N2.
From www.gkseries.com
In N molecule, the number of electrons shared by each nitrogen atom is What Is The Number Of Electrons Shared In N2 The total number of electrons present in the valence shell is 5 * 2 = 10e. Therefore, nitrogen molecule is formed by. It have 5 electron in its outer most shell. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve. What Is The Number Of Electrons Shared In N2.
From www.shutterstock.com
Nitrogen Atomic Structure Atomic Mass Atomic Stock Vector (Royalty Free What Is The Number Of Electrons Shared In N2 These are then paired up in an opposite spin (pauli exclusion principle). Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x. What Is The Number Of Electrons Shared In N2.
From askfilo.com
Which number of electrons shared by each outermost shell of N2 is [AIIM.. What Is The Number Of Electrons Shared In N2 These are then paired up in an opposite spin (pauli exclusion principle). In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x 2. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2. What Is The Number Of Electrons Shared In N2.
From chem.libretexts.org
Electron Configuration Chemistry LibreTexts What Is The Number Of Electrons Shared In N2 In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x 2. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). Total 6 electrons are shared in n₂. Each nitrogen atom. What Is The Number Of Electrons Shared In N2.
From topblogtenz.com
Nitrogen Orbital diagram, Electron configuration, Valence electrons What Is The Number Of Electrons Shared In N2 Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. Nitrogen is belong to group va. The total number of electrons present in the valence shell is 5 * 2 = 10e. Therefore, nitrogen molecule is formed by. Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?,. What Is The Number Of Electrons Shared In N2.
From www.alamy.com
3d render of atom structure of nitrogen isolated over white background What Is The Number Of Electrons Shared In N2 Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. Therefore, nitrogen molecule is formed by. Nitrogen is belong to group va. It have 5 electron in its outer most shell. Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is. What Is The Number Of Electrons Shared In N2.
From askfilo.com
In N2 molecule, the number of electrons shared by each nitrogen atom is.. What Is The Number Of Electrons Shared In N2 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. These are then paired up in an opposite spin (pauli exclusion principle). Thus, 10 valence electrons need to. What Is The Number Of Electrons Shared In N2.
From utedzz.blogspot.com
Nitrogen Periodic Table Protons Neutrons Electrons Periodic Table What Is The Number Of Electrons Shared In N2 These are then paired up in an opposite spin (pauli exclusion principle). Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. It have 5 electron in its outer most shell. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: Total 6 electrons are shared in n₂. 2 out. What Is The Number Of Electrons Shared In N2.
From chamotgallery.com
Nitrogen(N) electron configuration and orbital diagram (2022) What Is The Number Of Electrons Shared In N2 Each nitrogen atom has five valence electrons shared with another nitrogen atom in order to achieve a complete octet of eight valence. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: It have 5 electron in its outer most shell. 2 out of the 6 electrons are first singly filled in the π2p x and π2p. What Is The Number Of Electrons Shared In N2.
From www.slideserve.com
PPT Covalent Bonding and Nomenclature PowerPoint Presentation, free What Is The Number Of Electrons Shared In N2 Study with quizlet and memorize flashcards containing terms like which substance has nonpolar covalent bonds?, what is the number. In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x 2. Total 6 electrons are shared in n₂. Therefore, nitrogen molecule is formed by. These. What Is The Number Of Electrons Shared In N2.
From iperiodictable.com
Orbital Diagram For Nitrogen (N) Nitrogen Electron Configuration What Is The Number Of Electrons Shared In N2 These are then paired up in an opposite spin (pauli exclusion principle). It have 5 electron in its outer most shell. The total number of electrons present in the valence shell is 5 * 2 = 10e. Nitrogen is belong to group va. In this problem, we need to find the number of electrons shared between the atoms in a. What Is The Number Of Electrons Shared In N2.
From www.youtube.com
Number of unpaired electrons in N2+ is/are YouTube What Is The Number Of Electrons Shared In N2 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). Nitrogen is belong to group va. It have 5 electron in its outer most shell. Nitrogen molecule is diatomic and contains a triple bond between two n atoms: Each nitrogen atom has five valence electrons shared with. What Is The Number Of Electrons Shared In N2.
From mavink.com
Lewis Electron Dot Diagram Nitrogen What Is The Number Of Electrons Shared In N2 The total number of electrons present in the valence shell is 5 * 2 = 10e. These are then paired up in an opposite spin (pauli exclusion principle). In this problem, we need to find the number of electrons shared between the atoms in a molecule of nitrogen, n x 2 \ce{n2} n x 2. It have 5 electron in. What Is The Number Of Electrons Shared In N2.
From kdi-ppi.com
The Nitrogen Electron Shell Diagram Exploring Its Atomic Structure What Is The Number Of Electrons Shared In N2 Total 6 electrons are shared in n₂. 2 out of the 6 electrons are first singly filled in the π2p x and π2p y mos of n 2 (hund’s rule). Thus, 10 valence electrons need to be arranged in the structure to show the chemical bonding between. Each nitrogen atom has five valence electrons shared with another nitrogen atom in. What Is The Number Of Electrons Shared In N2.