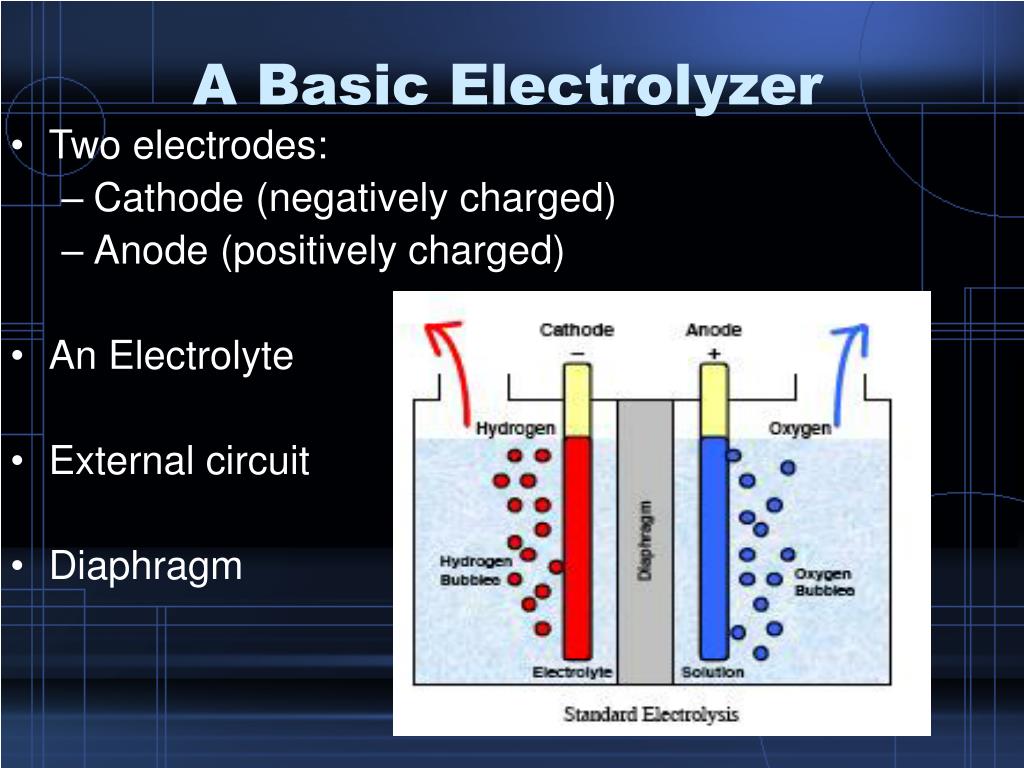
Function Of Electrodes In Electrolysis . a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). in electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an otherwise non. Ionic compounds conduct electricity when molten or in solution. reactive metals are extracted from their ores using electrolysis. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. the electrode definition is as follows: Two electrical conductors (electrodes) are. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when.
from www.slideserve.com
the electrode definition is as follows: Two electrical conductors (electrodes) are. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. reactive metals are extracted from their ores using electrolysis. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). Ionic compounds conduct electricity when molten or in solution. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. reactive metals are extracted from their ores using electrolysis. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. Ionic compounds conduct electricity when.
PPT Electrolysis PowerPoint Presentation, free download ID3366458
Function Of Electrodes In Electrolysis a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when. Two electrical conductors (electrodes) are. Ionic compounds conduct electricity when molten or in solution. in electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an otherwise non. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. the electrode definition is as follows: a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from their ores using electrolysis. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Function Of Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. in electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an. Function Of Electrodes In Electrolysis.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts Function Of Electrodes In Electrolysis reactive metals are extracted from their ores using electrolysis. reactive metals are extracted from their ores using electrolysis. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. Ionic compounds conduct electricity when molten or in solution. Two. Function Of Electrodes In Electrolysis.
From www.youtube.com
Chemistry Class 10 Electrolysis Principles of Electroplating YouTube Function Of Electrodes In Electrolysis Ionic compounds conduct electricity when. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. reactive metals are extracted from their ores using electrolysis. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. in electrolysis,. Function Of Electrodes In Electrolysis.
From en.ppt-online.org
Electrolysis online presentation Function Of Electrodes In Electrolysis reactive metals are extracted from their ores using electrolysis. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. Ionic compounds conduct electricity when. Ionic compounds conduct electricity when. Function Of Electrodes In Electrolysis.
From uen.pressbooks.pub
Electrolysis Introductory Chemistry Function Of Electrodes In Electrolysis the electrode definition is as follows: reactive metals are extracted from their ores using electrolysis. in electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an otherwise non. a familiar example of electrolysis is recharging a battery, which involves use of an. Function Of Electrodes In Electrolysis.
From fixlibrarylorierik8.z14.web.core.windows.net
Cathode Charge In Electrolysis Function Of Electrodes In Electrolysis The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from their. Function Of Electrodes In Electrolysis.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Function Of Electrodes In Electrolysis in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. reactive metals are extracted from their ores using electrolysis. in electrolysis, an electric current it sent. Function Of Electrodes In Electrolysis.
From socratic.org
Electrolysis Chemistry Socratic Function Of Electrodes In Electrolysis in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted. Function Of Electrodes In Electrolysis.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog Function Of Electrodes In Electrolysis reactive metals are extracted from their ores using electrolysis. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from their ores using electrolysis. Ionic compounds. Function Of Electrodes In Electrolysis.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Function Of Electrodes In Electrolysis Two electrical conductors (electrodes) are. in electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an otherwise non. reactive metals are extracted from their ores using electrolysis. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. a. Function Of Electrodes In Electrolysis.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an Function Of Electrodes In Electrolysis Ionic compounds conduct electricity when. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). Ionic compounds conduct electricity when molten or in solution. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. in electrolytic cells, electrical energy causes nonspontaneous. Function Of Electrodes In Electrolysis.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science Function Of Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. reactive metals are extracted from their ores using electrolysis. reactive metals are extracted from their ores using electrolysis. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). Ionic. Function Of Electrodes In Electrolysis.
From www.snexplores.org
Explainer What is an electrode? Function Of Electrodes In Electrolysis reactive metals are extracted from their ores using electrolysis. reactive metals are extracted from their ores using electrolysis. Two electrical conductors (electrodes) are. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. Ionic compounds conduct electricity when molten or in solution. . Function Of Electrodes In Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Function Of Electrodes In Electrolysis Two electrical conductors (electrodes) are. reactive metals are extracted from their ores using electrolysis. the electrode definition is as follows: The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. Ionic compounds conduct electricity when molten or in solution. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity. Function Of Electrodes In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Function Of Electrodes In Electrolysis in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. Ionic compounds conduct. Function Of Electrodes In Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Function Of Electrodes In Electrolysis a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). Two electrical conductors (electrodes) are. the electrode definition is as follows: The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when. reactive metals are extracted. Function Of Electrodes In Electrolysis.
From exovumdtp.blob.core.windows.net
How Is Electrolysis Done at Margaret Newman blog Function Of Electrodes In Electrolysis in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). in electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an otherwise non. Ionic compounds. Function Of Electrodes In Electrolysis.
From www.slideserve.com
PPT CHAPTER 6 ELECTROLYSIS PowerPoint Presentation, free download Function Of Electrodes In Electrolysis reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from their ores using electrolysis. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. Two electrical conductors (electrodes) are.. Function Of Electrodes In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Function Of Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. Two electrical conductors (electrodes) are. reactive metals are extracted from their ores using electrolysis. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. reactive metals are extracted from their ores using electrolysis. in electrolysis, an electric current it sent through an electrolyte. Function Of Electrodes In Electrolysis.
From circuitlibrarybauer.z13.web.core.windows.net
Strong Electrolyte Diagram Function Of Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. reactive metals are extracted from their ores using electrolysis. in electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an otherwise non. Ionic compounds conduct electricity when. in electrolytic cells, electrical energy causes nonspontaneous. Function Of Electrodes In Electrolysis.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki Function Of Electrodes In Electrolysis Ionic compounds conduct electricity when. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. reactive metals are extracted from their ores using electrolysis. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. reactive metals are extracted. Function Of Electrodes In Electrolysis.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Function Of Electrodes In Electrolysis The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. the electrode definition is as follows: reactive metals are extracted from their ores using electrolysis. in electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an otherwise non.. Function Of Electrodes In Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Function Of Electrodes In Electrolysis in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from their ores using electrolysis. Two electrical conductors (electrodes) are. a familiar example of electrolysis is recharging a battery, which involves use of an. Function Of Electrodes In Electrolysis.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte Function Of Electrodes In Electrolysis in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. Two electrical conductors (electrodes) are. reactive metals are extracted from their ores using electrolysis. reactive metals are extracted from their ores using electrolysis. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). a familiar example of. Function Of Electrodes In Electrolysis.
From semcouniversity.com
How the three electrode system works Semco University Semco Function Of Electrodes In Electrolysis a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. Two electrical conductors (electrodes) are. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. reactive metals are extracted from their ores using electrolysis. a typical. Function Of Electrodes In Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID4966899 Function Of Electrodes In Electrolysis reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). The electrode is an electrical conductor that. Function Of Electrodes In Electrolysis.
From www.researchgate.net
Schematic of the membrane electrode assembly (MEA) and catalyst Function Of Electrodes In Electrolysis reactive metals are extracted from their ores using electrolysis. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. Ionic compounds conduct electricity when molten or in solution. Two electrical conductors (electrodes) are. in electrolysis, an electric current it sent. Function Of Electrodes In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Function Of Electrodes In Electrolysis a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when. the electrode definition is as follows:. Function Of Electrodes In Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Function Of Electrodes In Electrolysis Ionic compounds conduct electricity when. Ionic compounds conduct electricity when molten or in solution. the electrode definition is as follows: reactive metals are extracted from their ores using electrolysis. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). . Function Of Electrodes In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID3366458 Function Of Electrodes In Electrolysis Two electrical conductors (electrodes) are. reactive metals are extracted from their ores using electrolysis. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. reactive metals are extracted. Function Of Electrodes In Electrolysis.
From saylordotorg.github.io
Electrolysis Function Of Electrodes In Electrolysis a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). in electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an otherwise non. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. Ionic compounds. Function Of Electrodes In Electrolysis.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Function Of Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. the electrode definition is as follows: reactive metals are extracted from their ores using electrolysis. The electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. Ionic compounds. Function Of Electrodes In Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Function Of Electrodes In Electrolysis Two electrical conductors (electrodes) are. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from their ores using electrolysis. a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. The electrode is an electrical conductor that. Function Of Electrodes In Electrolysis.
From circuitagolopetzin.z4.web.core.windows.net
What Happens At The Cathode In Electrolysis Function Of Electrodes In Electrolysis Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when. reactive metals are extracted from their ores using electrolysis. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from their ores using electrolysis. in electrolysis, an electric current it sent through an electrolyte and into. Function Of Electrodes In Electrolysis.
From kayleyewabarr.blogspot.com
Anode and Cathode in Electrolysis KayleyewaBarr Function Of Electrodes In Electrolysis a familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction. in electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an otherwise non. reactive metals are extracted from their. Function Of Electrodes In Electrolysis.