Microbial Ingress Testing . Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. Prevention of product formulation loss. The mall is based on product quality requirements. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. Prevention of microbial ingress to ensure product sterility.
from eduinput.com
This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. The mall is based on product quality requirements. Prevention of microbial ingress to ensure product sterility. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Prevention of product formulation loss. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow.
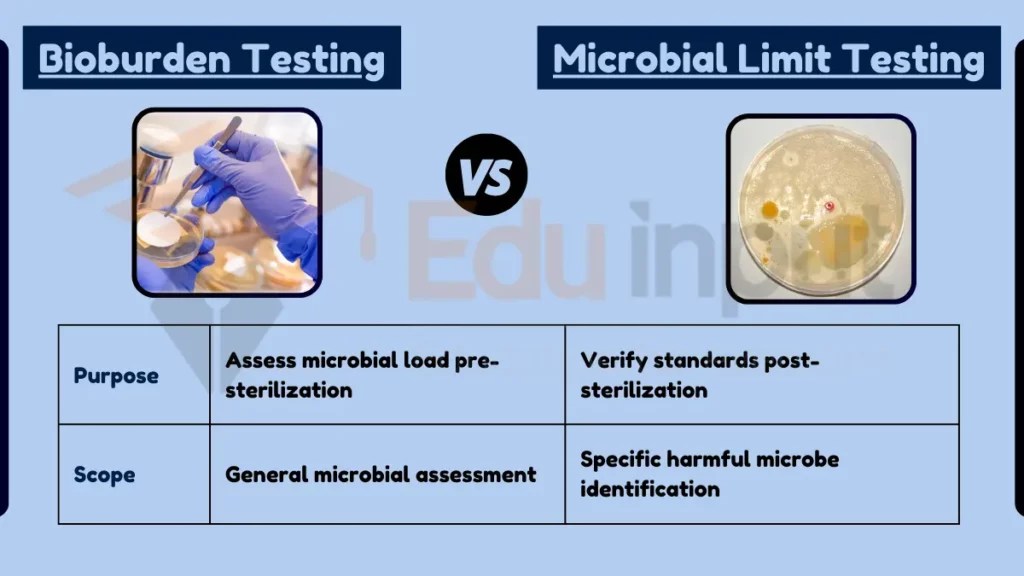
What is the Difference Between Bioburden and Microbial Limit Test?
Microbial Ingress Testing This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. Prevention of product formulation loss. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Prevention of microbial ingress to ensure product sterility. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. The mall is based on product quality requirements.
From wickhammicro.co.uk
Microbial Identification Wickham Micro Microbial Ingress Testing Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. Prevention of microbial ingress to ensure product. Microbial Ingress Testing.
From www.slideshare.net
Amatsigroup Infographics Microbial Testing for Quality Control Microbial Ingress Testing The mall is based on product quality requirements. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. This guidance document serves to assist manufacturers in the development. Microbial Ingress Testing.
From www.researchgate.net
(PDF) Microbial Ingress through Breaches in Aseptic Manufacturing Microbial Ingress Testing The mall is based on product quality requirements. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. An aerosol. Microbial Ingress Testing.
From twitter.com
Sartorius on Twitter "Check this white paper where we demonstrate the Microbial Ingress Testing Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. The mall is based on product quality requirements. Prevention of product formulation loss. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus. Microbial Ingress Testing.
From cptclabs.com
Reliable Microbial Limits Test CPT Labs Microbial Ingress Testing This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the. Microbial Ingress Testing.
From www.foodprocessing.com
The ABCs of Microbial Testing Food Processing Microbial Ingress Testing The mall is based on product quality requirements. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. An aerosol microbial ingress test was specifically designed and used to create. Microbial Ingress Testing.
From natsep.co.za
Microbial Testing Natsep Microbial Ingress Testing Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Test articles bearing calibrated defects over. Microbial Ingress Testing.
From www.scribd.com
SingleUse System Integrity I Using A Microbial Ingress Test Method To Microbial Ingress Testing An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. The mall is based on product quality requirements. Prevention of product formulation loss. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. Test articles bearing calibrated. Microbial Ingress Testing.
From pacificbiolabs.com
Microbial Limits Test Pacific BioLabs Microbial Ingress Testing Prevention of microbial ingress to ensure product sterility. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. Prevention of product formulation loss. An aerosol microbial ingress test was specifically designed and. Microbial Ingress Testing.
From www.pda.org
Microbial Ingress No Longer an Effective CCI Test Method PDA Microbial Ingress Testing This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. Prevention of product formulation loss. The mall is based on product quality requirements. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. Microbial exposure is achieved either by directly placing a. Microbial Ingress Testing.
From microbe-investigations.com
USP 61 Testing for Microbial Enumeration Microbe Investigations Microbial Ingress Testing Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. The mall is based on product quality requirements. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. This guidance document serves to assist manufacturers in the development. Microbial Ingress Testing.
From www.researchgate.net
(PDF) Microbial ingress through breaches in aseptic manufacturing Microbial Ingress Testing Prevention of microbial ingress to ensure product sterility. The mall is based on product quality requirements. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. An aerosol microbial ingress test was. Microbial Ingress Testing.
From etrlabs.com
The Importance of Microbial Testing for Your Industry ETR Microbial Ingress Testing Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. Prevention of microbial ingress to ensure product sterility. An aerosol microbial ingress test was specifically designed and used to create a predictive. Microbial Ingress Testing.
From journal.pda.org
SingleUse System Integrity I Using a Microbial Ingress Test Method to Microbial Ingress Testing This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. Prevention of product formulation loss. The mall is based on product quality requirements. Microbial exposure is achieved either by directly placing. Microbial Ingress Testing.
From wickhammicro.co.uk
Microbial Ingress Testing Wickham Micro Microbial Ingress Testing An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. Prevention of microbial ingress to ensure product sterility. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. The mall is based on product quality requirements. Test. Microbial Ingress Testing.
From www.pda.org
Microbial Ingress No Longer an Effective CCI Test Method PDA Microbial Ingress Testing Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. Prevention of product formulation loss. Prevention of microbial ingress to ensure product sterility. The mall is based on product quality requirements. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine. Microbial Ingress Testing.
From microchemlab.com
Testing Services Microchem Laboratory Microbial Ingress Testing Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. Prevention of microbial ingress to ensure product sterility. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow.. Microbial Ingress Testing.
From www.researchgate.net
(PDF) SingleUse System Integrity I Using a Microbial Ingress Test Microbial Ingress Testing Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. This guidance document serves to assist. Microbial Ingress Testing.
From www.a3p.org
Container Closure Integrity Testing of Sterile Injectable Products Microbial Ingress Testing An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Prevention of product formulation loss. Prevention of microbial ingress to ensure product sterility. The mall is based on product quality requirements. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected. Microbial Ingress Testing.
From mycoscience.com
MycoScience Microbial Aerosol Challenge Testing vs. Microbial Microbial Ingress Testing An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Prevention of microbial ingress to ensure product sterility. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. This guidance document serves to assist manufacturers in the. Microbial Ingress Testing.
From eduinput.com
What is the Difference Between Bioburden and Microbial Limit Test? Microbial Ingress Testing An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to.. Microbial Ingress Testing.
From www.mirri.org
Microbial growthpromoting and antimicrobial tests MIRRIERIC Microbial Ingress Testing An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. Prevention of product formulation loss. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. This guidance. Microbial Ingress Testing.
From sanatron.com
Dye Ingress Test inside a Transparent Test Chamber Microbial Ingress Testing An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. This guidance document serves to assist. Microbial Ingress Testing.
From www.pda.org
Microbial Ingress No Longer an Effective CCI Test Method PDA Microbial Ingress Testing The mall is based on product quality requirements. Prevention of microbial ingress to ensure product sterility. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. Prevention of product formulation loss. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine. Microbial Ingress Testing.
From journal.pda.org
SingleUse System Integrity I Using a Microbial Ingress Test Method to Microbial Ingress Testing This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. Prevention of product formulation loss. An aerosol microbial ingress test. Microbial Ingress Testing.
From www.biosafe.fi
Microbial testing An overview of what it is and what it is for Microbial Ingress Testing Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. Prevention of microbial ingress to ensure product sterility. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the. Microbial Ingress Testing.
From www.ajronline.org
Development of a New Imaging Bulk Package for Multipatient Use in MDCT Microbial Ingress Testing Prevention of product formulation loss. The mall is based on product quality requirements. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is. Microbial Ingress Testing.
From wickhammicro.co.uk
Microbial Environmental Monitoring Wickham Micro Microbial Ingress Testing An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Prevention of microbial ingress to ensure product sterility. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow. The mall is based on product quality requirements. Prevention of. Microbial Ingress Testing.
From www.equashield.com
A 7 Days Microbial Ingress Test of Single Use Vials Utilizing the Microbial Ingress Testing Prevention of product formulation loss. Prevention of microbial ingress to ensure product sterility. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. An aerosol microbial ingress test was specifically designed and used to create a predictive model. Microbial Ingress Testing.
From mandflab.com
Microbial Testing Service Medical and Food Lab Co., Ltd. Microbial Ingress Testing Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. An aerosol microbial ingress test was specifically designed and used. Microbial Ingress Testing.
From www.researchgate.net
(PDF) SingleUse System Integrity I Using a Microbial Ingress Test Microbial Ingress Testing Prevention of product formulation loss. Prevention of microbial ingress to ensure product sterility. This guidance document serves to assist manufacturers in the development of appropriate test methods, types of microorganisms to. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. An aerosol microbial ingress test was specifically. Microbial Ingress Testing.
From microbiologyguideritesh.com
Flow Chart of Microbial Limit Test Microbiology Guidelines Microbial Ingress Testing Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. Prevention of microbial ingress to ensure product. Microbial Ingress Testing.
From www.youtube.com
Difference Between BIOBURDEN TEST AND MICROBIAL LIMIT TEST YouTube Microbial Ingress Testing An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum. Prevention of product formulation loss. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Test articles bearing calibrated defects over a range of dimensions, including up. Microbial Ingress Testing.
From www.slideshare.net
Microbial limit tests I.P By Dr.P.Srinivas Jangaon institute of pharm… Microbial Ingress Testing Prevention of microbial ingress to ensure product sterility. The mall is based on product quality requirements. Prevention of product formulation loss. An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution,. Microbial Ingress Testing.
From journal.pda.org
SingleUse System Integrity I Using a Microbial Ingress Test Method to Microbial Ingress Testing An aerosol microbial ingress test was specifically designed and used to create a predictive model in order to determine the maximum allowable. Microbial exposure is achieved either by directly placing a sus into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a sus that is placed inside a. Prevention of microbial ingress to ensure. Microbial Ingress Testing.