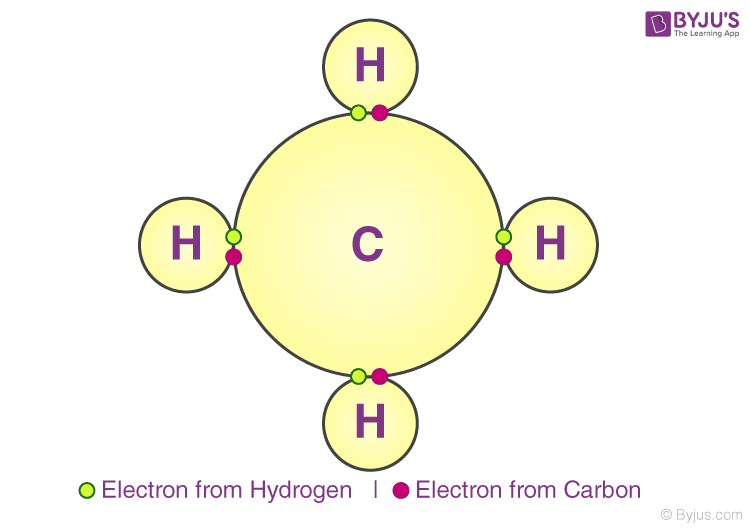
What Are The Three Types Of Bonds In Chemistry . A bond between two atoms depends upon the. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. There are 4 primary types of chemical. — the type of chemical bonds formed varies in strength and properties. — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. all models of chemical bonding have three common features: An ionic bond is formed when one atom accepts. Atoms form bonds because the products are more stable. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. — do you know how? An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another.
from byjus.com
— do you know how? There are 4 primary types of chemical. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. Atoms form bonds because the products are more stable. An ionic bond is formed when one atom accepts. — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. all models of chemical bonding have three common features: chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. — the type of chemical bonds formed varies in strength and properties.
Chemical Bonding Types of Chemical Bonds, Bond Characteristics, Enthalpy
What Are The Three Types Of Bonds In Chemistry — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. Atoms form bonds because the products are more stable. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. — do you know how? An ionic bond is formed when one atom accepts. — the type of chemical bonds formed varies in strength and properties. There are 4 primary types of chemical. all models of chemical bonding have three common features: chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. A bond between two atoms depends upon the.
From www.indiapicturebudget.com
Chemical Bonds infographic diagram showing types of bonding including What Are The Three Types Of Bonds In Chemistry An ionic bond is formed when one atom accepts. all models of chemical bonding have three common features: the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. — do you know how? There are 4 primary types of chemical. — the two main types of bonds formed between. What Are The Three Types Of Bonds In Chemistry.
From printablejuliocbgov.z21.web.core.windows.net
Summarize The 3 Types Of Bonds What Are The Three Types Of Bonds In Chemistry — the type of chemical bonds formed varies in strength and properties. There are 4 primary types of chemical. — do you know how? Atoms form bonds because the products are more stable. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. An ionic bond is formed when one. What Are The Three Types Of Bonds In Chemistry.
From www.ownguru.com
concept of Chemical bond What Are The Three Types Of Bonds In Chemistry — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. — do you know how? — the type of chemical bonds formed varies in strength and properties. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. There are 4 primary types of. What Are The Three Types Of Bonds In Chemistry.
From sciencenotes.org
Types of Chemical Bonds What Are The Three Types Of Bonds In Chemistry A bond between two atoms depends upon the. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. — do you know how? chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. An ionic bond. What Are The Three Types Of Bonds In Chemistry.
From learningschooldsbbbb56.z4.web.core.windows.net
Types Of Chemical Bonds Chemistry What Are The Three Types Of Bonds In Chemistry all models of chemical bonding have three common features: — do you know how? Atoms form bonds because the products are more stable. There are 4 primary types of chemical. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. — the two main types of bonds formed between. What Are The Three Types Of Bonds In Chemistry.
From mavink.com
Covalent Bond Types What Are The Three Types Of Bonds In Chemistry all models of chemical bonding have three common features: — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. — do you know how? An ionic bond is formed when one atom accepts.. What Are The Three Types Of Bonds In Chemistry.
From learningschooldsbbbb56.z4.web.core.windows.net
Summarize The 3 Types Of Bonds What Are The Three Types Of Bonds In Chemistry chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to. What Are The Three Types Of Bonds In Chemistry.
From www.slideserve.com
PPT Types of chemical bonds PowerPoint Presentation, free download What Are The Three Types Of Bonds In Chemistry — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. — the type of chemical bonds formed varies in strength and properties. Atoms form bonds because the products are more stable. all models of chemical bonding have three common features: chemical bonds form when electrons can be simultaneously close to. What Are The Three Types Of Bonds In Chemistry.
From www.youtube.com
Chemistry Lesson 31 Different Types of Chemical Bonds YouTube What Are The Three Types Of Bonds In Chemistry Atoms form bonds because the products are more stable. — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. — the type of chemical bonds formed varies in strength and properties. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no. What Are The Three Types Of Bonds In Chemistry.
From www.youtube.com
Types of Bonding (Ionic, Covalent, Metallic) GCSE Chemistry Revision What Are The Three Types Of Bonds In Chemistry all models of chemical bonding have three common features: — the type of chemical bonds formed varies in strength and properties. A bond between two atoms depends upon the. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. — do you. What Are The Three Types Of Bonds In Chemistry.
From dxomztejs.blob.core.windows.net
What Are The Three Types Of Bonding In Chemistry at Andrea Hoeppner blog What Are The Three Types Of Bonds In Chemistry — the type of chemical bonds formed varies in strength and properties. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. — do you know how? An ionic bond is formed when one atom accepts. A bond between two atoms depends upon the. There are 4 primary types of. What Are The Three Types Of Bonds In Chemistry.
From www.slideserve.com
PPT IV. Chemical Bonding PowerPoint Presentation, free download ID What Are The Three Types Of Bonds In Chemistry — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another.. What Are The Three Types Of Bonds In Chemistry.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint What Are The Three Types Of Bonds In Chemistry There are 4 primary types of chemical. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. the main types of chemical bonds are ionic bond,. What Are The Three Types Of Bonds In Chemistry.
From www.britannica.com
chemical bonding Definition, Types, & Examples Britannica What Are The Three Types Of Bonds In Chemistry An ionic bond is formed when one atom accepts. all models of chemical bonding have three common features: chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. An atom can form chemical bonds in three ways, (a) by losing one or more electrons. What Are The Three Types Of Bonds In Chemistry.
From byjus.com
Chemical Bonding Types of Chemical Bonds, Bond Characteristics, Enthalpy What Are The Three Types Of Bonds In Chemistry There are 4 primary types of chemical. — the type of chemical bonds formed varies in strength and properties. — do you know how? A bond between two atoms depends upon the. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. Atoms form bonds because the products are more. What Are The Three Types Of Bonds In Chemistry.
From dxomztejs.blob.core.windows.net
What Are The Three Types Of Bonding In Chemistry at Andrea Hoeppner blog What Are The Three Types Of Bonds In Chemistry the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. all models of chemical bonding have three common features: An ionic bond is formed when one atom accepts. — do you know. What Are The Three Types Of Bonds In Chemistry.
From www.slideserve.com
PPT Types of chemical bonds PowerPoint Presentation, free download What Are The Three Types Of Bonds In Chemistry There are 4 primary types of chemical. A bond between two atoms depends upon the. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. Atoms form. What Are The Three Types Of Bonds In Chemistry.
From biologydictionary.net
Covalent Bond Biology Dictionary What Are The Three Types Of Bonds In Chemistry — do you know how? An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. Atoms form bonds because the products are more stable. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. A bond. What Are The Three Types Of Bonds In Chemistry.
From www.slideserve.com
PPT CHEMICAL BONDS PowerPoint Presentation, free download ID6445592 What Are The Three Types Of Bonds In Chemistry Atoms form bonds because the products are more stable. — do you know how? A bond between two atoms depends upon the. all models of chemical bonding have three common features: There are 4 primary types of chemical. — the type of chemical bonds formed varies in strength and properties. An ionic bond is formed when one. What Are The Three Types Of Bonds In Chemistry.
From www.phdnest.com
Covalent Bond Meaning, Types, Properties, Examples PhD Nest What Are The Three Types Of Bonds In Chemistry — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. all models of chemical bonding have three common features: A bond between two atoms depends upon the. An ionic bond is formed when one. What Are The Three Types Of Bonds In Chemistry.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples What Are The Three Types Of Bonds In Chemistry A bond between two atoms depends upon the. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. Atoms form bonds because the products are more stable. — do you know how? There are 4 primary types of chemical. An ionic bond is formed. What Are The Three Types Of Bonds In Chemistry.
From www.slideshare.net
10/26 What are the three types of chemical bonds? Part II What Are The Three Types Of Bonds In Chemistry chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. — do you know how? There are 4 primary types of chemical. A bond between two. What Are The Three Types Of Bonds In Chemistry.
From inovasibiologi.com
Ikatan Kimia INOVASI BIOLOGI What Are The Three Types Of Bonds In Chemistry — do you know how? Atoms form bonds because the products are more stable. A bond between two atoms depends upon the. There are 4 primary types of chemical. An ionic bond is formed when one atom accepts. all models of chemical bonding have three common features: — the type of chemical bonds formed varies in strength. What Are The Three Types Of Bonds In Chemistry.
From www.dreamstime.com
Three Types of Covalent Bonds Including Single, Double, and Triple What Are The Three Types Of Bonds In Chemistry all models of chemical bonding have three common features: — the type of chemical bonds formed varies in strength and properties. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. An ionic bond is formed when one atom accepts. A bond between. What Are The Three Types Of Bonds In Chemistry.
From studylib.net
Types of Chemical Bonds What Are The Three Types Of Bonds In Chemistry the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. An ionic bond is formed when one atom accepts. A bond between two atoms depends upon the. all models of chemical bonding have three common features: — the two main types of bonds formed between atoms are ionic bonds and. What Are The Three Types Of Bonds In Chemistry.
From learningschooldsbbbb56.z4.web.core.windows.net
Describe The Three Types Of Chemical Bonds What Are The Three Types Of Bonds In Chemistry An ionic bond is formed when one atom accepts. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. — do you know how? — the type of chemical bonds formed varies in strength and properties. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond,. What Are The Three Types Of Bonds In Chemistry.
From asoefkersaachemistry.weebly.com
Different Types of Bonds Chemistry What Are The Three Types Of Bonds In Chemistry — do you know how? An ionic bond is formed when one atom accepts. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. A bond between two atoms depends upon the. There are 4 primary types of chemical. — the type of. What Are The Three Types Of Bonds In Chemistry.
From worksheetlibreminds.z13.web.core.windows.net
Summarize The 3 Types Of Bonds What Are The Three Types Of Bonds In Chemistry Atoms form bonds because the products are more stable. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. A bond between two atoms depends upon the. There are 4 primary types of chemical. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2].. What Are The Three Types Of Bonds In Chemistry.
From learningschoolgroenselex.z4.web.core.windows.net
Types Of Chemical Bonds Explained What Are The Three Types Of Bonds In Chemistry the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. all models of chemical bonding have three common features: — do you know how? An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. Atoms form bonds because the products are more. What Are The Three Types Of Bonds In Chemistry.
From madoverchemistry.com
54. Types of bonds, Ionic bonds. Madoverchemistry What Are The Three Types Of Bonds In Chemistry A bond between two atoms depends upon the. — do you know how? An ionic bond is formed when one atom accepts. all models of chemical bonding have three common features: — the type of chemical bonds formed varies in strength and properties. — the two main types of bonds formed between atoms are ionic bonds. What Are The Three Types Of Bonds In Chemistry.
From learningschooljakobi97.z14.web.core.windows.net
Types Of Chemical Bonds Chemistry What Are The Three Types Of Bonds In Chemistry Atoms form bonds because the products are more stable. An ionic bond is formed when one atom accepts. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. — do you know how? A bond between two atoms depends upon the. — the type of chemical bonds formed varies in. What Are The Three Types Of Bonds In Chemistry.
From exomswreo.blob.core.windows.net
Three Different Types Of Side Bonds Are at Carol Carver blog What Are The Three Types Of Bonds In Chemistry An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. What Are The Three Types Of Bonds In Chemistry.
From www.slideserve.com
PPT Mastering Chemistry PowerPoint Presentation, free download ID What Are The Three Types Of Bonds In Chemistry all models of chemical bonding have three common features: An ionic bond is formed when one atom accepts. There are 4 primary types of chemical. — do you know how? chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. Atoms form bonds. What Are The Three Types Of Bonds In Chemistry.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples What Are The Three Types Of Bonds In Chemistry — the two main types of bonds formed between atoms are ionic bonds and covalent bonds. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2].. What Are The Three Types Of Bonds In Chemistry.
From www.biorender.com
Types of Chemical Bonds BioRender Science Templates What Are The Three Types Of Bonds In Chemistry all models of chemical bonding have three common features: the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. An atom can form chemical bonds in three ways, (a) by losing one or more electrons to another. There are 4 primary types of chemical. chemical bonds form when electrons can. What Are The Three Types Of Bonds In Chemistry.