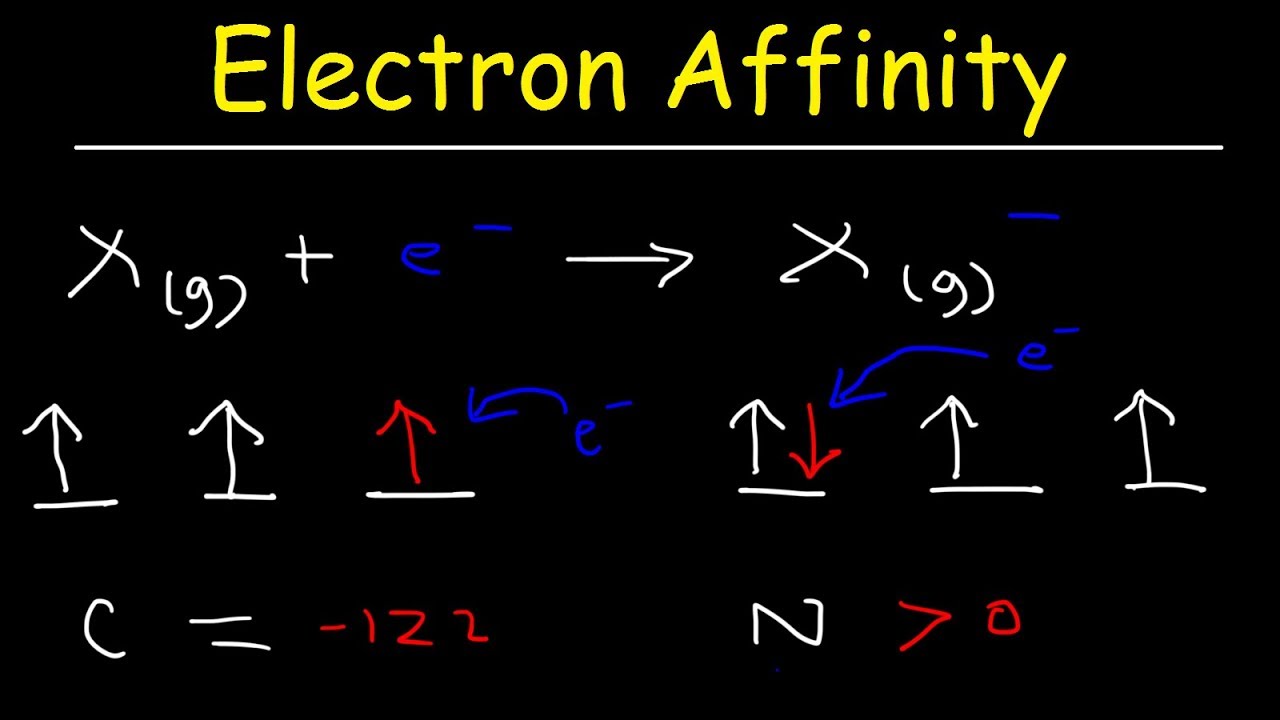
Is Electron Affinity Negative . the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. definition of electron affinity. The energy released is 349 kj/mol. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. since energy is released, the electron affinity values are negative. Basically, energy is required in order to attach an electron. Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest. E l e c t r o n a f f i n i t y e q u a t i o n. Higher negative values indicate a stable anion. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is.
from apomillionaire.weebly.com
E l e c t r o n a f f i n i t y e q u a t i o n. The energy released is 349 kj/mol. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. Higher negative values indicate a stable anion. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. since energy is released, the electron affinity values are negative. definition of electron affinity.
Electron affinity chart apomillionaire
Is Electron Affinity Negative since energy is released, the electron affinity values are negative. Higher negative values indicate a stable anion. Basically, energy is required in order to attach an electron. in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. since energy is released, the electron affinity values are negative. definition of electron affinity. the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. E l e c t r o n a f f i n i t y e q u a t i o n. Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. The energy released is 349 kj/mol.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Is Electron Affinity Negative Higher negative values indicate a stable anion. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. since energy is released, the electron affinity values are negative. Basically, energy is required in order to attach an electron. E l e c t r o n a f f i. Is Electron Affinity Negative.
From www.pearson.com
The periodic table with electron affinity values is shown below Is Electron Affinity Negative Basically, energy is required in order to attach an electron. definition of electron affinity. E l e c t r o n a f f i n i t y e q u a t i o n. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. The. Is Electron Affinity Negative.
From www.periodictableprintable.com
Most Negative Electron Affinity Periodic Table 2024 Periodic Table Is Electron Affinity Negative Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. since energy is released, the electron affinity values are negative. the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. Higher negative values indicate a stable anion. E l e c t r o. Is Electron Affinity Negative.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download Is Electron Affinity Negative since energy is released, the electron affinity values are negative. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. The energy released is 349 kj/mol. definition of electron affinity. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron. Is Electron Affinity Negative.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID2938613 Is Electron Affinity Negative Basically, energy is required in order to attach an electron. The energy released is 349 kj/mol. in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. E l e c t r o n a f f i n i t y e q u a t i o n.. Is Electron Affinity Negative.
From www.breakingatom.com
Electron Affinity of The Elements Is Electron Affinity Negative Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. E l e c t r o n a f f i n i t y e q u a t i o n. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. in. Is Electron Affinity Negative.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Is Electron Affinity Negative the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. Higher negative values indicate a stable anion. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. E l e c t r o n a f f i n i. Is Electron Affinity Negative.
From periodictable.thesetupwarrior.com
Electron Affinity Periodic Table Periodic Trends Electron Affinity You Is Electron Affinity Negative since energy is released, the electron affinity values are negative. in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest. Basically, energy is required in order to attach an electron. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that. Is Electron Affinity Negative.
From knordslearning.com
Electron Affinity Chart of All Elements (With Periodic Table) Is Electron Affinity Negative The energy released is 349 kj/mol. in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. Higher negative values indicate a stable anion. since energy is released, the electron affinity. Is Electron Affinity Negative.
From www.breakingatom.com
Electron Affinity of The Elements Is Electron Affinity Negative unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. . Is Electron Affinity Negative.
From www.slideshare.net
APChem Chapter 7 Lecture Periodic Trends Is Electron Affinity Negative Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest. . Is Electron Affinity Negative.
From lillybutler.z13.web.core.windows.net
Negative Electron Affinity Chart Is Electron Affinity Negative Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. The energy released is 349 kj/mol. since energy is released, the electron affinity values are negative. in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. unlike electronegativity,. Is Electron Affinity Negative.
From chem.libretexts.org
7.5 Electron Affinities Chemistry LibreTexts Is Electron Affinity Negative in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. The energy released is 349 kj/mol. in general, elements with the most negative electron affinities (the highest affinity for. Is Electron Affinity Negative.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Is Electron Affinity Negative in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. The energy released is 349 kj/mol. since energy is released, the electron affinity values are negative. definition of electron affinity. Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. Higher negative values. Is Electron Affinity Negative.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Is Electron Affinity Negative Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. Basically, energy is required in order to attach an electron. since energy is released, the electron affinity values are negative. the change in energy δe has a positive sign and the electron affinity e ea has a negative. Is Electron Affinity Negative.
From studyandanswers.com
The most negative electron affinity is most likely associated with Is Electron Affinity Negative E l e c t r o n a f f i n i t y e q u a t i o n. in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest. Higher negative values indicate a stable anion. unlike electronegativity, electron. Is Electron Affinity Negative.
From study.com
Electron Affinity Definition, Trends & Equation Video & Lesson Is Electron Affinity Negative definition of electron affinity. in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest. Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. Electron affinity is a quantitative measurement of the energy change that occurs when an electron. Is Electron Affinity Negative.
From unilasopa610.weebly.com
What is electron affinity unilasopa Is Electron Affinity Negative The energy released is 349 kj/mol. definition of electron affinity. since energy is released, the electron affinity values are negative. in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest. E l e c t r o n a f f i n. Is Electron Affinity Negative.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Is Electron Affinity Negative in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest.. Is Electron Affinity Negative.
From chemistnotes.com
Electron Affinity Definition, Trends, and Equation Chemistry Notes Is Electron Affinity Negative The energy released is 349 kj/mol. the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. Basically, energy is required in order to attach an electron. E l e c t r o n a f f i n i t y e q u a t i o n.. Is Electron Affinity Negative.
From www.slideserve.com
PPT Chapter 4 The Periodic Table PowerPoint Presentation ID6474399 Is Electron Affinity Negative the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. The energy released is 349 kj/mol. E l e c t r o n a f f i n i. Is Electron Affinity Negative.
From www.numerade.com
SOLVED Electron affinity, EA, is the energy required to add an Is Electron Affinity Negative Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. Higher negative values indicate a stable anion. Basically, energy is required in order to attach an electron. definition of electron affinity. E l e. Is Electron Affinity Negative.
From www.youtube.com
Electron affinity period trend Atomic structure and properties AP Is Electron Affinity Negative Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. Higher negative values indicate a stable anion. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. in chemistry, electron affinity simply means the energy that is released when an electron is added to a. Is Electron Affinity Negative.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Is Electron Affinity Negative in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest. the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs. Is Electron Affinity Negative.
From apomillionaire.weebly.com
Electron affinity chart apomillionaire Is Electron Affinity Negative Basically, energy is required in order to attach an electron. in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. since energy is released, the. Is Electron Affinity Negative.
From www.slideserve.com
PPT Chapter 8 Periodic Properties PowerPoint Presentation, free Is Electron Affinity Negative unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. Basically,. Is Electron Affinity Negative.
From www.pinterest.com
Electron Affinity Trend and Definition Electron affinity, Electrons Is Electron Affinity Negative Higher negative values indicate a stable anion. definition of electron affinity. Basically, energy is required in order to attach an electron. Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. E l e c t r o n a f f i n i t y e q u a t i o. Is Electron Affinity Negative.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2834450 Is Electron Affinity Negative Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral. Higher negative values indicate a stable anion. since energy is released, the electron affinity values are negative. definition of electron affinity. the change in energy δe has a positive sign and the electron affinity e ea has. Is Electron Affinity Negative.
From pubchem.ncbi.nlm.nih.gov
Electron Affinity Periodic Table of Elements PubChem Is Electron Affinity Negative Basically, energy is required in order to attach an electron. the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. since energy is released, the electron affinity values are negative. The energy released is 349 kj/mol. Higher negative values indicate a stable anion. E l e c t r. Is Electron Affinity Negative.
From chem.libretexts.org
2.10 Electron Affinities Chemistry LibreTexts Is Electron Affinity Negative in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest. definition of electron affinity. Higher negative values indicate a stable anion. the change in energy δe has a positive sign and the electron affinity e ea has a negative sign. since energy. Is Electron Affinity Negative.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Is Electron Affinity Negative Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. E l e c t r o n a f f i n i t y e q u a t i o n. definition of electron affinity. since energy is released, the electron affinity values are negative. Basically, energy is required in. Is Electron Affinity Negative.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Is Electron Affinity Negative in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. definition of electron affinity. Higher. Is Electron Affinity Negative.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint Is Electron Affinity Negative in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. since energy is released, the electron affinity values are negative. Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. unlike electronegativity, electron affinity is a quantitative measurement of the energy change that. Is Electron Affinity Negative.
From periodictableguide.com
Electron Affinity Chart (Labeled Periodic table + List) Is Electron Affinity Negative unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is. Basically, energy is required in order to attach an electron. Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. in chemistry, electron affinity simply means the energy that is released when an electron is. Is Electron Affinity Negative.
From www.slideserve.com
PPT Electron affinity PowerPoint Presentation, free download ID3704050 Is Electron Affinity Negative Electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. in chemistry, electron affinity simply means the energy that is released when an electron is added to a neutral. in general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest.. Is Electron Affinity Negative.