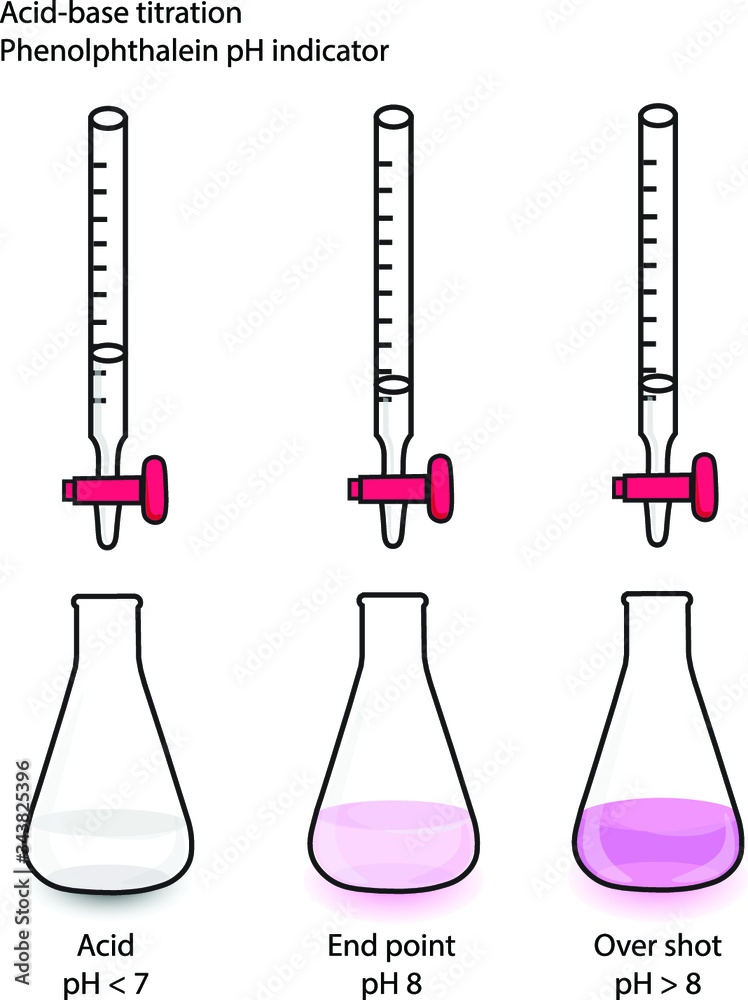
Phenolphthalein Why Is It A Good Indicator . In this case, the weak acid is colourless and its ion is bright pink. In this case, the weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid.
from www.vrogue.co
In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between.
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co
Phenolphthalein Why Is It A Good Indicator How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Phenolphthalein Why Is It A Good Indicator We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. How does the ph at the equivalence point, as well as the ph range. Phenolphthalein Why Is It A Good Indicator.
From www.alamy.com
Phenolphthalein acidbase indicator. Phenolphthalein indicator changes Phenolphthalein Why Is It A Good Indicator In this case, the weak acid is colourless and its ion is bright pink. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein An Appropriate Indicator For T vrogue.co Phenolphthalein Why Is It A Good Indicator We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at. Phenolphthalein Why Is It A Good Indicator.
From www.youtube.com
Titration end point using phenolphthalein indicator YouTube Phenolphthalein Why Is It A Good Indicator In this case, the weak acid. In this case, the weak acid is colourless and its ion is bright pink. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. Phenolphthalein is another commonly used indicator for titrations, and is another. Phenolphthalein Why Is It A Good Indicator.
From www.priyamstudycentre.com
Phenolphthalein Indicator, Solution, Uses Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid. In this case, the weak acid is colourless and its ion is bright pink. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for. Phenolphthalein Why Is It A Good Indicator.
From www.toppr.com
tion 11 Phenolphthalein is not a good indicator titrating NaOH against Phenolphthalein Why Is It A Good Indicator We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. In this case, the weak acid is colourless and its ion is bright pink. How does the ph at the equivalence point, as well as the ph range over which the. Phenolphthalein Why Is It A Good Indicator.
From www.indiamart.com
Phenolphthalein Indicator 125ml, For Used As Orp Solution, Grade Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. We will have to add. Phenolphthalein Why Is It A Good Indicator.
From www.youtube.com
Acid Base Indicator Phenolphthalein explained with experiment Phenolphthalein Why Is It A Good Indicator How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak. Phenolphthalein Why Is It A Good Indicator.
From www.youtube.com
Why Phenolphthalein indicator color changes to pink. YouTube Phenolphthalein Why Is It A Good Indicator How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Phenolphthalein Why Is It A Good Indicator We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. Phenolphthalein is. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Phenolphthalein Why Is It A Good Indicator In this case, the weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is. Phenolphthalein Why Is It A Good Indicator.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C030/7311 Science Photo Library Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. In this case, the weak acid. In this case, the weak acid is colourless and its ion is. Phenolphthalein Why Is It A Good Indicator.
From www.macsenlab.com
Phenolphthalein Indicator 77098 Manufacturer & Supplier Phenolphthalein Why Is It A Good Indicator In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. We will have to add an excess of naoh (aq) to the. Phenolphthalein Why Is It A Good Indicator.
From thechemistrynotes.com
Phenolphthalein Indicator Synthesis, Uses, Properties, Preparation Phenolphthalein Why Is It A Good Indicator In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. How does the ph. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Phenolphthalein Why Is It A Good Indicator In this case, the weak acid is colourless and its ion is bright pink. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak. Phenolphthalein Why Is It A Good Indicator.
From www.youtube.com
How to make a Phenolphthalein Indicator Solution (0.05wt) YouTube Phenolphthalein Why Is It A Good Indicator We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at. Phenolphthalein Why Is It A Good Indicator.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation ID4827855 Phenolphthalein Why Is It A Good Indicator We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour. Phenolphthalein Why Is It A Good Indicator.
From www.numerade.com
SOLVED Explain why phenolphthalein is a good indicator for the Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak. Phenolphthalein Why Is It A Good Indicator.
From sciencenotes.org
Phenolphthalein Indicator Phenolphthalein Why Is It A Good Indicator In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for. Phenolphthalein Why Is It A Good Indicator.
From www.youtube.com
Phenolphthalein Indicator preparation how to make w/v phenolphthalein Phenolphthalein Why Is It A Good Indicator In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. In this case, the. Phenolphthalein Why Is It A Good Indicator.
From www.britannica.com
Phenolphthalein pH indicator, acidbase titration, indicator dye Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by. Phenolphthalein Why Is It A Good Indicator.
From www.youtube.com
Phenolphthalein Indicator Viva Questions pH range and Chemical Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at. Phenolphthalein Why Is It A Good Indicator.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C039/1215 Science Photo Library Phenolphthalein Why Is It A Good Indicator We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. In this case, the weak acid is colourless and its ion is bright pink. How does the ph at the equivalence point, as well as the ph range over which the. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Phenolphthalein Why Is It A Good Indicator How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. Phenolphthalein is. Phenolphthalein Why Is It A Good Indicator.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. How does the ph at the equivalence point, as well as the ph range over which the colour. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. In this case, the weak. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Phenolphthalein Why Is It A Good Indicator We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. How does the ph at the equivalence point, as well as the. Phenolphthalein Why Is It A Good Indicator.
From www.youtube.com
How to make 1 Phenolphthalein Indicator YouTube Phenolphthalein Why Is It A Good Indicator In this case, the weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point. Phenolphthalein Why Is It A Good Indicator.
From www.rcilabscan.com
Phenolphthalein Indicator, AR RCI LABSCAN LIMITED (EN) Phenolphthalein Why Is It A Good Indicator In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. How does the ph at the equivalence point, as well as the. Phenolphthalein Why Is It A Good Indicator.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C039/1218 Science Photo Library Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. In this case, the weak acid is colourless and its ion is. Phenolphthalein Why Is It A Good Indicator.
From www.amazon.in
Phenolphthalein Indicator Solution 125ML Amazon.in Industrial Phenolphthalein Why Is It A Good Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. We will have to add an excess of naoh (aq) to the hcl (aq) to make phenolphthalein change colour, in other words, the end point as indicated by the. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly. Phenolphthalein Why Is It A Good Indicator.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Phenolphthalein Why Is It A Good Indicator In this case, the weak acid. How does the ph at the equivalence point, as well as the ph range over which the colour of phenolphthalein changes, make it a suitable indicator for titrations between. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is. Phenolphthalein Why Is It A Good Indicator.