Potassium And Zinc Chloride Balanced Equation . K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. Zncl2 + k2co3 = kcl + znco3 is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and one. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balanced chemical equations have the same number and type of each atom on both. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. This equation is not balanced, and is therefore not a valid chemical equation. In order to balance this equation, we must insert coefficients (not. To be useful, chemical equations must always be balanced. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced equation will appear.
from www.numerade.com
To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. To be useful, chemical equations must always be balanced. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced equation will appear. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. This equation is not balanced, and is therefore not a valid chemical equation. In order to balance this equation, we must insert coefficients (not. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Balanced chemical equations have the same number and type of each atom on both.
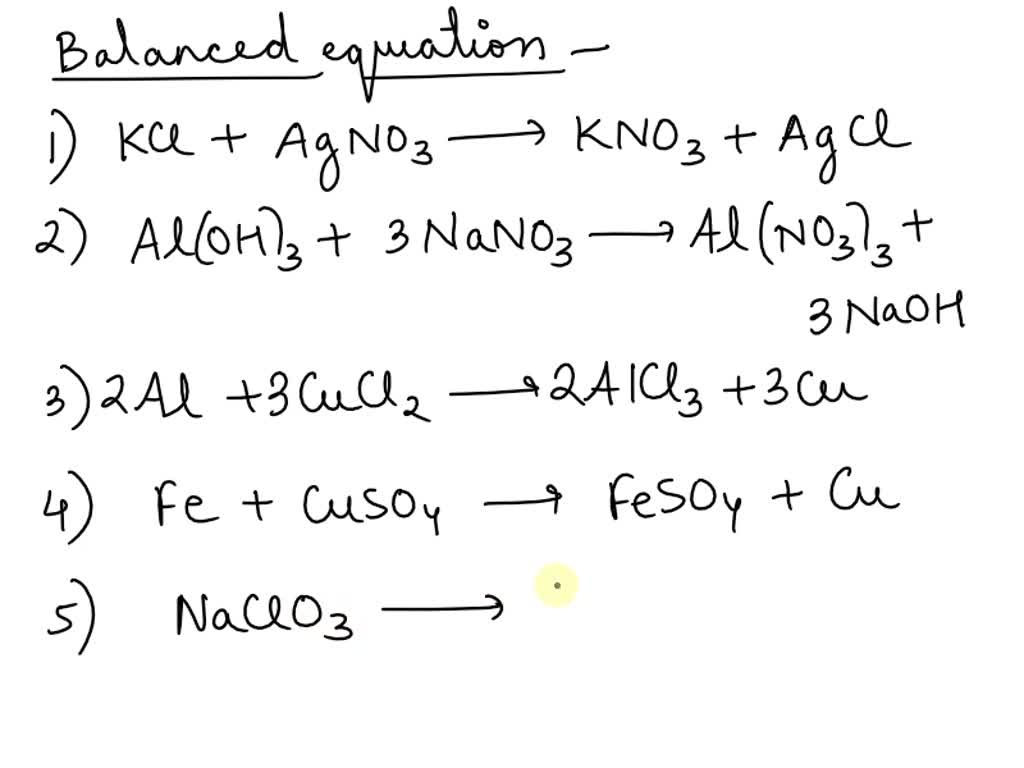
SOLVED Write balanced equations for each of the following reactions
Potassium And Zinc Chloride Balanced Equation Balanced chemical equations have the same number and type of each atom on both. This equation is not balanced, and is therefore not a valid chemical equation. In order to balance this equation, we must insert coefficients (not. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Zncl2 + k2co3 = kcl + znco3 is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and one. The balanced equation will appear. Balanced chemical equations have the same number and type of each atom on both. To be useful, chemical equations must always be balanced. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple.
From www.transtutors.com
(Solved) The Reaction Of Zinc Metal And Hydrochloric Acid Produces Potassium And Zinc Chloride Balanced Equation Zncl2 + k2co3 = kcl + znco3 is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and one. To be useful, chemical equations must always be balanced. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In order to balance this equation, we must insert coefficients. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED BALANCING CHEMICAL EQUATIONS HYDROGEN GAS OXYGEN GAS WATER Potassium And Zinc Chloride Balanced Equation To be useful, chemical equations must always be balanced. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple. Balanced chemical equations have the same number and type of each atom on both.. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED Write the balanced molecular, ionic, and net ionic equations Potassium And Zinc Chloride Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. This equation is not balanced, and is therefore not a valid chemical equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In order to balance this equation, we. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED 5 . Predict the products of the following reactions and balance Potassium And Zinc Chloride Balanced Equation This equation is not balanced, and is therefore not a valid chemical equation. Zncl2 + k2co3 = kcl + znco3 is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and one. To be useful, chemical equations must always be balanced. The balanced equation will appear. Balanced chemical equations have the same number and type. Potassium And Zinc Chloride Balanced Equation.
From www.toppr.com
Write the balanced chemical equation the following reactions & identify Potassium And Zinc Chloride Balanced Equation Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The balanced equation will appear. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. A balanced chemical equation often may be derived from a qualitative description. Potassium And Zinc Chloride Balanced Equation.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation, free Potassium And Zinc Chloride Balanced Equation Balanced chemical equations have the same number and type of each atom on both. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. This equation is not balanced, and is therefore not a valid chemical equation. A balanced chemical equation often may be derived from a. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVEDWhen potassium chlorate is subjected to high temperatures, it Potassium And Zinc Chloride Balanced Equation A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple. To be useful, chemical equations must always be balanced. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. Zncl2 + k2co3 = kcl. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED Write balanced equations for each of the following reactions Potassium And Zinc Chloride Balanced Equation The balanced equation will appear. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. In order to balance this equation, we must insert coefficients (not. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. K. Potassium And Zinc Chloride Balanced Equation.
From www.slideshare.net
Chemical Reactions Potassium And Zinc Chloride Balanced Equation To be useful, chemical equations must always be balanced. This equation is not balanced, and is therefore not a valid chemical equation. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. Balanced chemical equations have the same number and type of each atom. Potassium And Zinc Chloride Balanced Equation.
From www.chegg.com
Solved 4. Write a balanced chemical equation for each of the Potassium And Zinc Chloride Balanced Equation Zncl2 + k2co3 = kcl + znco3 is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and one. The balanced equation will appear. Balanced chemical equations have the same number and type of each atom on both. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED 'What is the empirical formula of zinc chloride? Show Potassium And Zinc Chloride Balanced Equation Zncl2 + k2co3 = kcl + znco3 is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and one. To be useful, chemical equations must always be balanced. In order to balance this equation, we must insert coefficients (not. This equation is not balanced, and is therefore not a valid chemical equation. Balanced chemical equations. Potassium And Zinc Chloride Balanced Equation.
From www.slideserve.com
PPT Chapter 8 Chemical Equations and Reactions PowerPoint Potassium And Zinc Chloride Balanced Equation The balanced equation will appear. To be useful, chemical equations must always be balanced. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Balanced chemical equations. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the precipitation reaction that Potassium And Zinc Chloride Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced equation will appear. This equation is not balanced, and is therefore not a valid chemical equation. To be. Potassium And Zinc Chloride Balanced Equation.
From www.toppr.com
8. Write the balanced chemical equation the following and identify the Potassium And Zinc Chloride Balanced Equation Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. A balanced chemical equation often may be derived from a qualitative description of some. Potassium And Zinc Chloride Balanced Equation.
From studylib.net
Equations and Moles Potassium And Zinc Chloride Balanced Equation The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. To be useful, chemical equations must always be balanced. In order to balance this equation, we must insert coefficients (not. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element. Potassium And Zinc Chloride Balanced Equation.
From www.chemicals.co.uk
Practical GCSE Chemistry Making Salts The Science Blog Potassium And Zinc Chloride Balanced Equation K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. In order to balance this equation, we must insert coefficients (not. Zncl2 + k2co3 = kcl + znco3 is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and. Potassium And Zinc Chloride Balanced Equation.
From questions-in.kunduz.com
Word Equation Balanced Chemical Equation Z... Organic Chemistry Potassium And Zinc Chloride Balanced Equation K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. To be useful, chemical equations must always be balanced. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. To balance. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED Silver can be precipitated from a silver nitrate solution with Potassium And Zinc Chloride Balanced Equation A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple. The balanced equation will appear. This equation is not balanced, and is therefore not a valid chemical equation. Zncl2 + k2co3 = kcl + znco3 is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2]. Potassium And Zinc Chloride Balanced Equation.
From www.toppr.com
Write the balanced chemical equation the following and identify the of Potassium And Zinc Chloride Balanced Equation Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple. To be useful, chemical equations must always be balanced. The balanced equation will appear. In order to balance this equation,. Potassium And Zinc Chloride Balanced Equation.
From www.toppr.com
Write the balanced chemical equations for the following reactions.(a Potassium And Zinc Chloride Balanced Equation In order to balance this equation, we must insert coefficients (not. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. Balanced chemical equations have the same number and type of each atom on both. Zncl2 + k2co3 = kcl + znco3 is a. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED Write balanced equations for each of the following reactions Potassium And Zinc Chloride Balanced Equation To be useful, chemical equations must always be balanced. Balanced chemical equations have the same number and type of each atom on both. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. Limiting reagent can be computed for a balanced equation by entering. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the precipitation reaction that Potassium And Zinc Chloride Balanced Equation The balanced equation will appear. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. Zncl2 + k2co3 = kcl +. Potassium And Zinc Chloride Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for KCl + Pb(NO3)2 = KNO3 + PbCl2 Potassium And Zinc Chloride Balanced Equation K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In order to balance this equation, we must insert coefficients (not. Limiting. Potassium And Zinc Chloride Balanced Equation.
From www.toppr.com
Write balanced chemical equations for the following reactions(i) A Potassium And Zinc Chloride Balanced Equation This equation is not balanced, and is therefore not a valid chemical equation. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. Zncl2 + k2co3 = kcl + znco3 is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl. Potassium And Zinc Chloride Balanced Equation.
From schools.aglasem.com
NCERT Solutions for Class 10th Science Chapter 1 Chemical Reactions Potassium And Zinc Chloride Balanced Equation To be useful, chemical equations must always be balanced. This equation is not balanced, and is therefore not a valid chemical equation. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The balanced equation will appear. The chemical equation described in section 4.1 is balanced, meaning that equal numbers. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVEDWrite the balanced molecular equation for each of the chemical Potassium And Zinc Chloride Balanced Equation To be useful, chemical equations must always be balanced. This equation is not balanced, and is therefore not a valid chemical equation. In order to balance this equation, we must insert coefficients (not. Balanced chemical equations have the same number and type of each atom on both. A balanced chemical equation often may be derived from a qualitative description of. Potassium And Zinc Chloride Balanced Equation.
From www.toppr.com
Write the balanced chemical equations for the following reactions.(a Potassium And Zinc Chloride Balanced Equation Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. To be useful, chemical equations must always be balanced. In order to balance this equation, we must insert coefficients (not. The balanced equation will appear. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms. Potassium And Zinc Chloride Balanced Equation.
From questions.kunduz.com
Question 22 (2 points) Listen When potass... Physical Chemistry Potassium And Zinc Chloride Balanced Equation The balanced equation will appear. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. To balance a chemical equation, enter an equation of. Potassium And Zinc Chloride Balanced Equation.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Potassium And Zinc Chloride Balanced Equation This equation is not balanced, and is therefore not a valid chemical equation. Balanced chemical equations have the same number and type of each atom on both. The balanced equation will appear. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. K + zncl2 = kcl + zn is. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED A solution of potassium permanganate is mixed with zinc metal Potassium And Zinc Chloride Balanced Equation Zncl2 + k2co3 = kcl + znco3 is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and one. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This equation is not balanced, and is therefore not a valid chemical equation. The balanced equation will appear. A. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED Write balanced complete chemical equation the complete ionic Potassium And Zinc Chloride Balanced Equation To be useful, chemical equations must always be balanced. This equation is not balanced, and is therefore not a valid chemical equation. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. The chemical equation described in section 4.1 is balanced, meaning that equal. Potassium And Zinc Chloride Balanced Equation.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Potassium And Zinc Chloride Balanced Equation Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. In order to balance this equation, we must insert coefficients (not. This equation is not balanced, and is therefore not a valid chemical equation. Balanced chemical equations have the same number and type of each atom on both. The chemical. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equations for the following Potassium And Zinc Chloride Balanced Equation The balanced equation will appear. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple. To be useful, chemical equations must always be balanced. In order to balance this equation, we must insert coefficients (not. Balanced chemical equations have the same number and type of each atom on both. K. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced equation describing each of the following Potassium And Zinc Chloride Balanced Equation Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. To be useful, chemical equations must always be balanced. In order to balance this equation, we must. Potassium And Zinc Chloride Balanced Equation.
From www.numerade.com
SOLVED Write and balance this chemical equation zinc burns in oxygen Potassium And Zinc Chloride Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. K + zncl2 = kcl + zn is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of aqueous zinc. To be useful, chemical equations must always be balanced. In order to balance this equation, we must. Potassium And Zinc Chloride Balanced Equation.