Electrochemical Equilibrium Definition . at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. To understand the relationship between cell potential and the equilibrium constant. In this section we will introduce the electrochemical potential into the gibbs potential and. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any.
from www.studocu.com
In this section we will introduce the electrochemical potential into the gibbs potential and. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. To understand the relationship between cell potential and the equilibrium constant. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most.
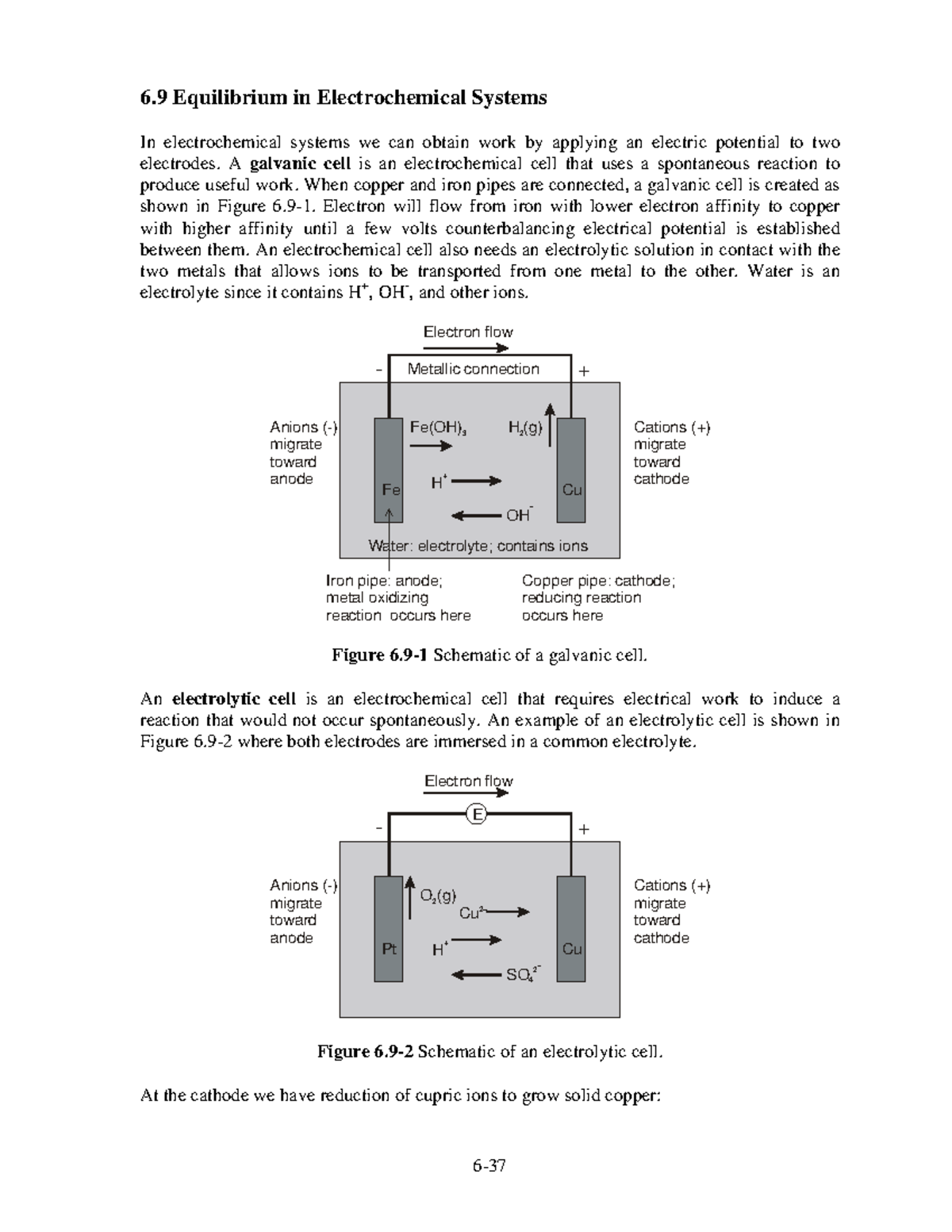
Chemistry47 Chemistry47 6 Equilibrium in Electrochemical Systems In electrochemical
Electrochemical Equilibrium Definition electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. To understand the relationship between cell potential and the equilibrium constant. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. In this section we will introduce the electrochemical potential into the gibbs potential and.
From www.youtube.com
Electrochemistry Equilibrium YouTube Electrochemical Equilibrium Definition “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. To understand the relationship between cell potential and the equilibrium constant. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential. Electrochemical Equilibrium Definition.
From www.studocu.com
Chemistry47 Chemistry47 6 Equilibrium in Electrochemical Systems In electrochemical Electrochemical Equilibrium Definition at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. To understand the relationship between cell potential and the equilibrium constant. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. electrochemical reactions can be analyzed using the gibbs free energy and. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT Equilibrium Electrochemistry PowerPoint Presentation, free download ID2338622 Electrochemical Equilibrium Definition at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. To. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT chap. 2 The resting membrane potential PowerPoint Presentation, free download ID9578669 Electrochemical Equilibrium Definition “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. In this section we will introduce the electrochemical potential into the gibbs potential and. To understand the relationship between cell potential and the equilibrium constant. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for. Electrochemical Equilibrium Definition.
From www.researchgate.net
Electrochemical equilibrium (EpH) diagram for Nb in water without... Download Scientific Diagram Electrochemical Equilibrium Definition In this section we will introduce the electrochemical potential into the gibbs potential and. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. “equilibrium electrochemistry, which is mainly based on equilibrium. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT ERT 108 PHYSICAL CHEMISTRY Equilibrium Electrochemistry PowerPoint Presentation ID4386046 Electrochemical Equilibrium Definition To understand the relationship between cell potential and the equilibrium constant. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. In this section we will introduce the electrochemical potential into the gibbs potential and. at. Electrochemical Equilibrium Definition.
From www.researchgate.net
Electrochemical Equilibria (A) Illustration of the energy landscape... Download Scientific Diagram Electrochemical Equilibrium Definition In this section we will introduce the electrochemical potential into the gibbs potential and. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. To understand the relationship between cell potential and. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT Equilibrium Electrochemistry PowerPoint Presentation, free download ID2338622 Electrochemical Equilibrium Definition In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. In this section we will introduce the electrochemical. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT Equilibrium Electrochemistry PowerPoint Presentation, free download ID2338622 Electrochemical Equilibrium Definition at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. In this section we will introduce the electrochemical potential into the gibbs potential and. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. To understand the relationship between cell potential and. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT Equilibrium Electrochemistry PowerPoint Presentation, free download ID2338622 Electrochemical Equilibrium Definition “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. In this section we will introduce the electrochemical potential into the gibbs potential and. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. In this section we will introduce the electrochemical potential into. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free download ID3358415 Electrochemical Equilibrium Definition “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. To understand the relationship between cell potential and the equilibrium constant. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. electrochemical reactions can be analyzed using the gibbs free energy and chemical. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT Electrochemical cells PowerPoint Presentation, free download ID1837944 Electrochemical Equilibrium Definition To understand the relationship between cell potential and the equilibrium constant. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. In this section we will introduce the electrochemical potential into the gibbs potential and. In this section we will introduce the electrochemical potential into the gibbs potential and. Electrochemical Equilibrium Definition.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Electrochemical Equilibrium Definition In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. In this section we will introduce the electrochemical potential into the gibbs potential and. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. at equilibrium, the electrochemical potential of any given species must. Electrochemical Equilibrium Definition.
From slideplayer.com
Ionic Generation of Electrical Signals ppt download Electrochemical Equilibrium Definition In this section we will introduce the electrochemical potential into the gibbs potential and. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. electrochemical reactions can be analyzed using the. Electrochemical Equilibrium Definition.
From slideplayer.com
Equilibrium Electrochemistry ppt download Electrochemical Equilibrium Definition electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. To understand the relationship between cell potential and the equilibrium constant. at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is. Electrochemical Equilibrium Definition.
From www.youtube.com
Further Physical Chemistry Electrochemistry session 6 SHORT YouTube Electrochemical Equilibrium Definition • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. To understand the relationship between cell potential and the equilibrium constant. at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. In this section we will introduce the electrochemical. Electrochemical Equilibrium Definition.
From www.studocu.com
1 Electro equilibrium and Nernst Equation Electrochemical Equilibrium and Nernst Equation Electrochemical Equilibrium Definition In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. To understand the relationship between cell potential and the equilibrium constant. at equilibrium, the electrochemical potential of any given species must. Electrochemical Equilibrium Definition.
From www.studocu.com
Chemical equilibrium part1 LESSON 1 ELECTROCHEMISTRY Chemical equilibrium Hydrogen and iodine Electrochemical Equilibrium Definition at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. In this section we will introduce the electrochemical potential into the gibbs potential and. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. electrochemical reactions can be analyzed using the gibbs. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free download ID3358415 Electrochemical Equilibrium Definition • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. In this section we. Electrochemical Equilibrium Definition.
From slideplayer.com
ERT 108 PHYSICAL CHEMISTRY Equilibrium Electrochemistry By; Mrs Haf iza Bint i Shu kor ERT 108 Electrochemical Equilibrium Definition To understand the relationship between cell potential and the equilibrium constant. at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. In this section we will introduce the electrochemical potential into the. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT Resting and action potential PowerPoint Presentation, free download ID5255179 Electrochemical Equilibrium Definition at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. “equilibrium. Electrochemical Equilibrium Definition.
From thechemistrynotes.com
Chemical Equilibrium Definition, Types, Importance, and Examples Electrochemical Equilibrium Definition In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. To understand the relationship between cell potential and the equilibrium constant. In this section we will introduce the electrochemical potential into the gibbs potential and. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. electrochemical reactions. Electrochemical Equilibrium Definition.
From www.youtube.com
ELECTROCHEMISTRY(NERNST EQUATION FOR EQUILIBRIUM CELL), CLASS12, LECTURE11 YouTube Electrochemical Equilibrium Definition “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. To understand the relationship between cell potential and the equilibrium constant. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of. Electrochemical Equilibrium Definition.
From itsreleased.com
Nernst Equation Understanding Electrochemical Equilibrium Electrochemical Equilibrium Definition In this section we will introduce the electrochemical potential into the gibbs potential and. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. “equilibrium electrochemistry, which is mainly based on. Electrochemical Equilibrium Definition.
From studylib.net
Electrochemical Equilibrium Electrochemical Equilibrium Definition electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. To understand the relationship between cell potential and the equilibrium constant. In this section we will introduce the electrochemical potential into the gibbs potential and. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. In this. Electrochemical Equilibrium Definition.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 10 simple voltaic cell YouTube Electrochemical Equilibrium Definition at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. In this section we will introduce the electrochemical potential into the gibbs potential and. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. In this section we will. Electrochemical Equilibrium Definition.
From www.youtube.com
Electrochemistry 10 Relationship between Nernst Equation and Equilibrium Constant (Kc) YouTube Electrochemical Equilibrium Definition To understand the relationship between cell potential and the equilibrium constant. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. at equilibrium, the electrochemical potential of any given species must be the. Electrochemical Equilibrium Definition.
From www.scribd.com
Electrochemical Equilibrium PDF Electrochemistry Dissociation (Chemistry) Electrochemical Equilibrium Definition To understand the relationship between cell potential and the equilibrium constant. In this section we will introduce the electrochemical potential into the gibbs potential and. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their.. Electrochemical Equilibrium Definition.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrochemical Equilibrium Definition electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. at equilibrium, the electrochemical potential. Electrochemical Equilibrium Definition.
From www.youtube.com
Electrochemistry Equilibrium, Free Energy, and Potential YouTube Electrochemical Equilibrium Definition at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. In this section we will introduce the electrochemical potential into the gibbs potential and. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. In this section we will. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Electrochemical Equilibrium Definition To understand the relationship between cell potential and the equilibrium constant. at equilibrium, the electrochemical potential of any given species must be the same throughout the system, and for any. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. • at equilibrium, the electrochemical potential of any given species must. Electrochemical Equilibrium Definition.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Electrochemical Equilibrium Definition • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. In this section we will introduce the electrochemical potential into the gibbs potential and. To understand the relationship between cell potential and the equilibrium constant. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the. Electrochemical Equilibrium Definition.
From www.numerade.com
SOLVED I. Electrochemical equilibrium potentials and membrane potential A. For the ion Electrochemical Equilibrium Definition electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. In this section we will introduce the electrochemical potential into the gibbs potential and discuss approaches to. “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. at equilibrium, the electrochemical potential of any given species. Electrochemical Equilibrium Definition.
From www.youtube.com
Electrochemistry Part6 Equilibrium Constant for Redox Equations YouTube Electrochemical Equilibrium Definition In this section we will introduce the electrochemical potential into the gibbs potential and. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. • at equilibrium, the electrochemical potential of any given species must be the same throughout the system • for any. To understand the relationship between cell potential. Electrochemical Equilibrium Definition.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID1075415 Electrochemical Equilibrium Definition “equilibrium electrochemistry, which is mainly based on equilibrium thermodynamics, is one of the most. In this section we will introduce the electrochemical potential into the gibbs potential and. electrochemical reactions can be analyzed using the gibbs free energy and chemical potential for determination of their. In this section we will introduce the electrochemical potential into the gibbs potential. Electrochemical Equilibrium Definition.