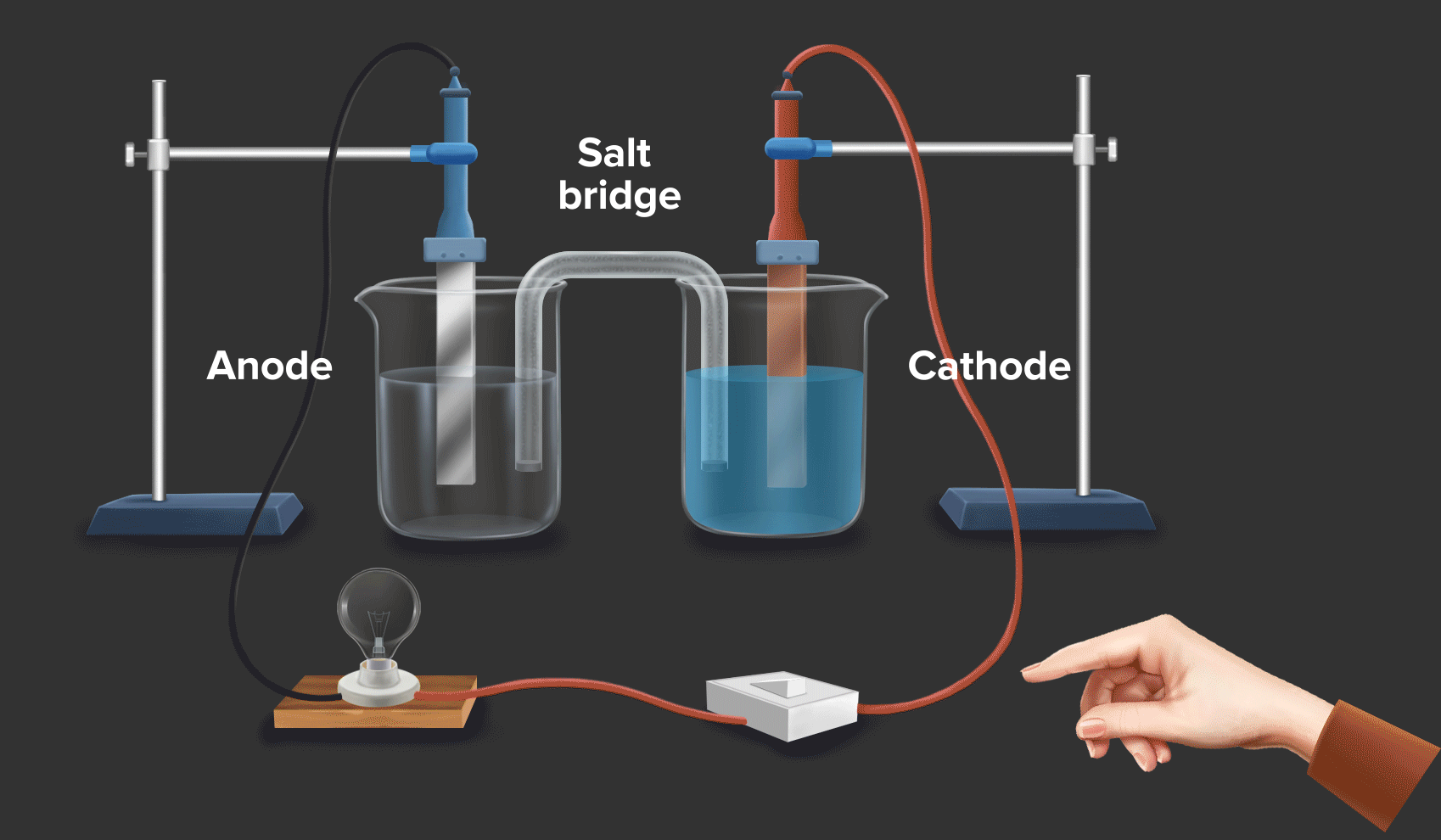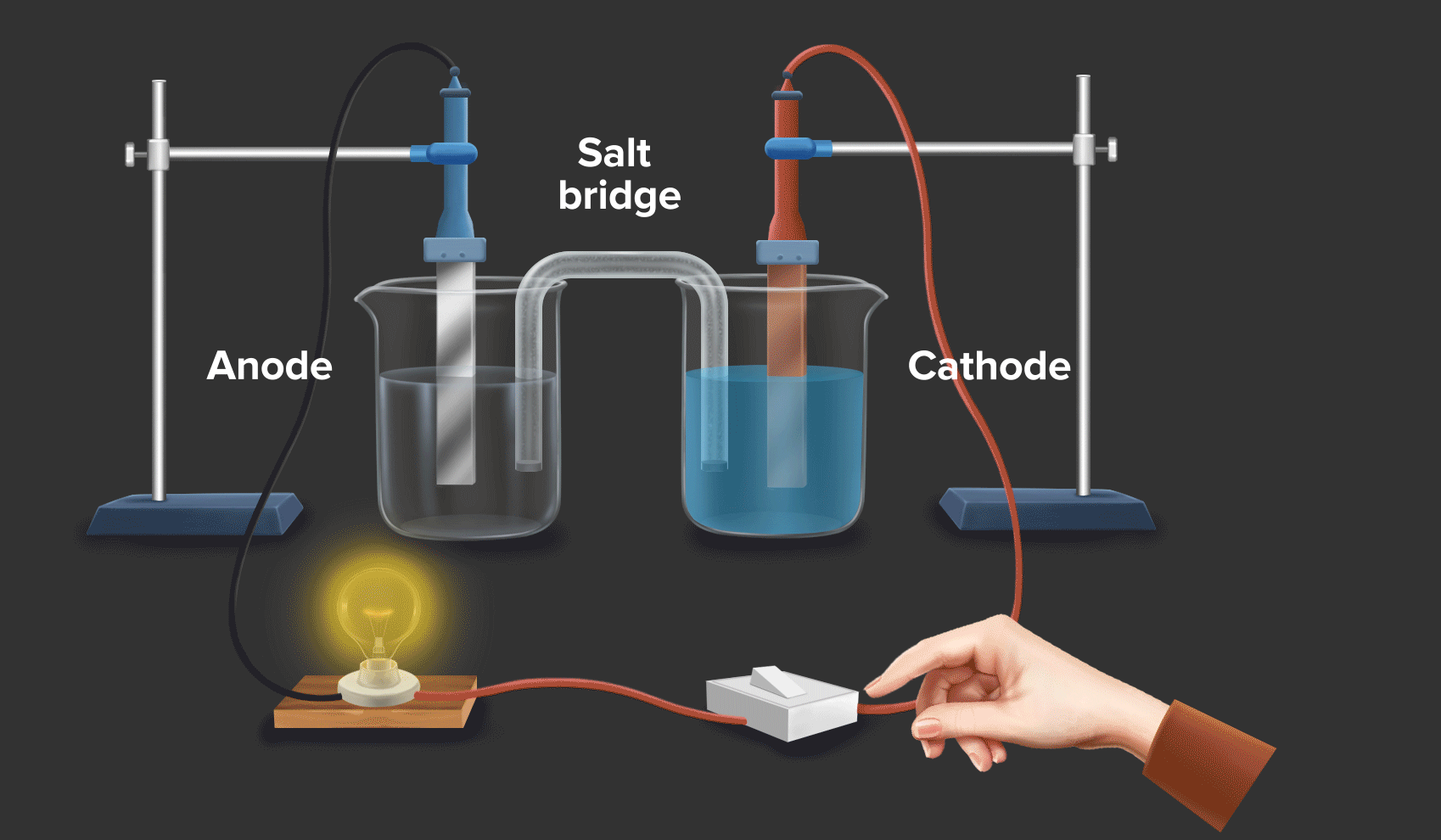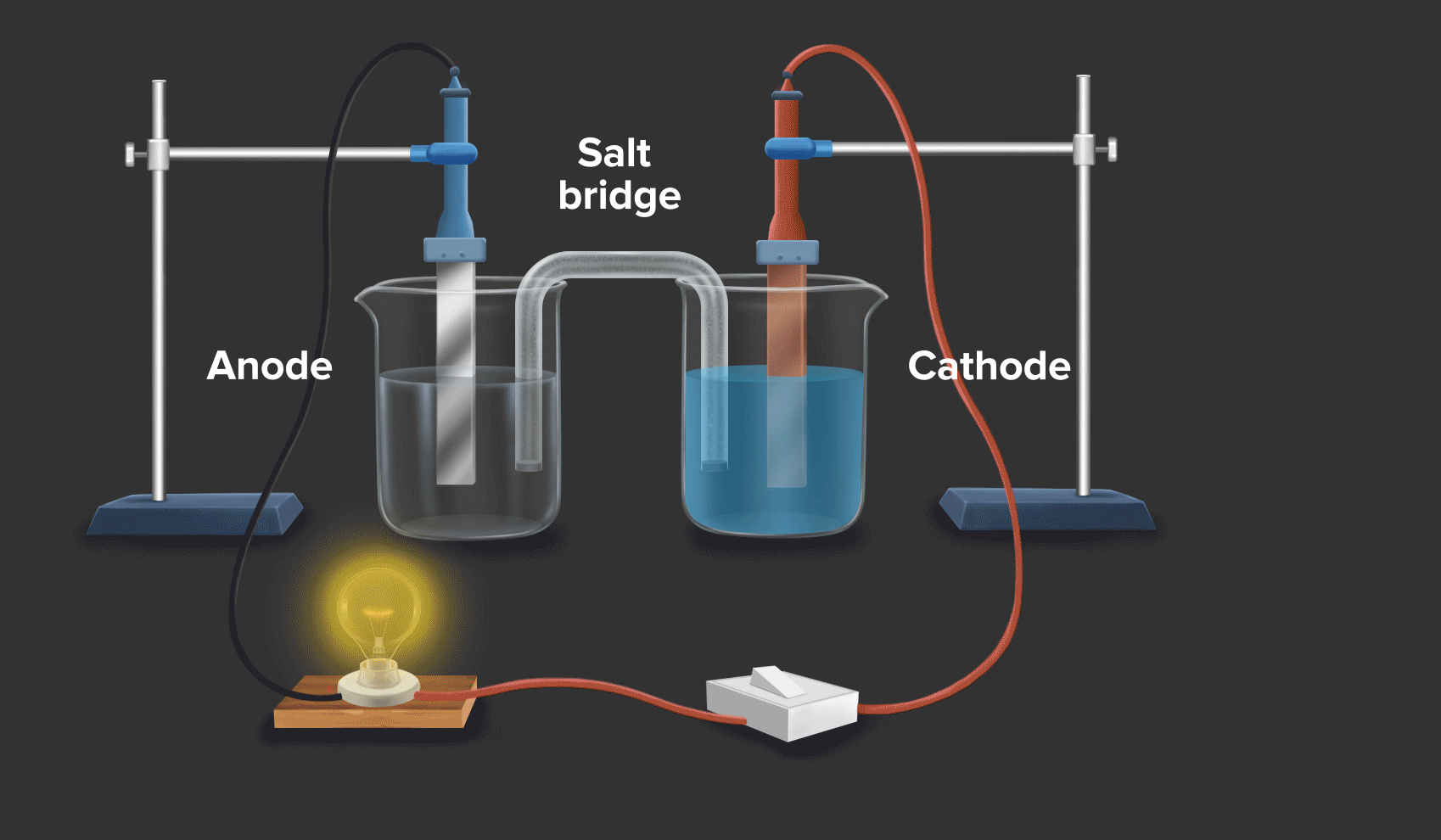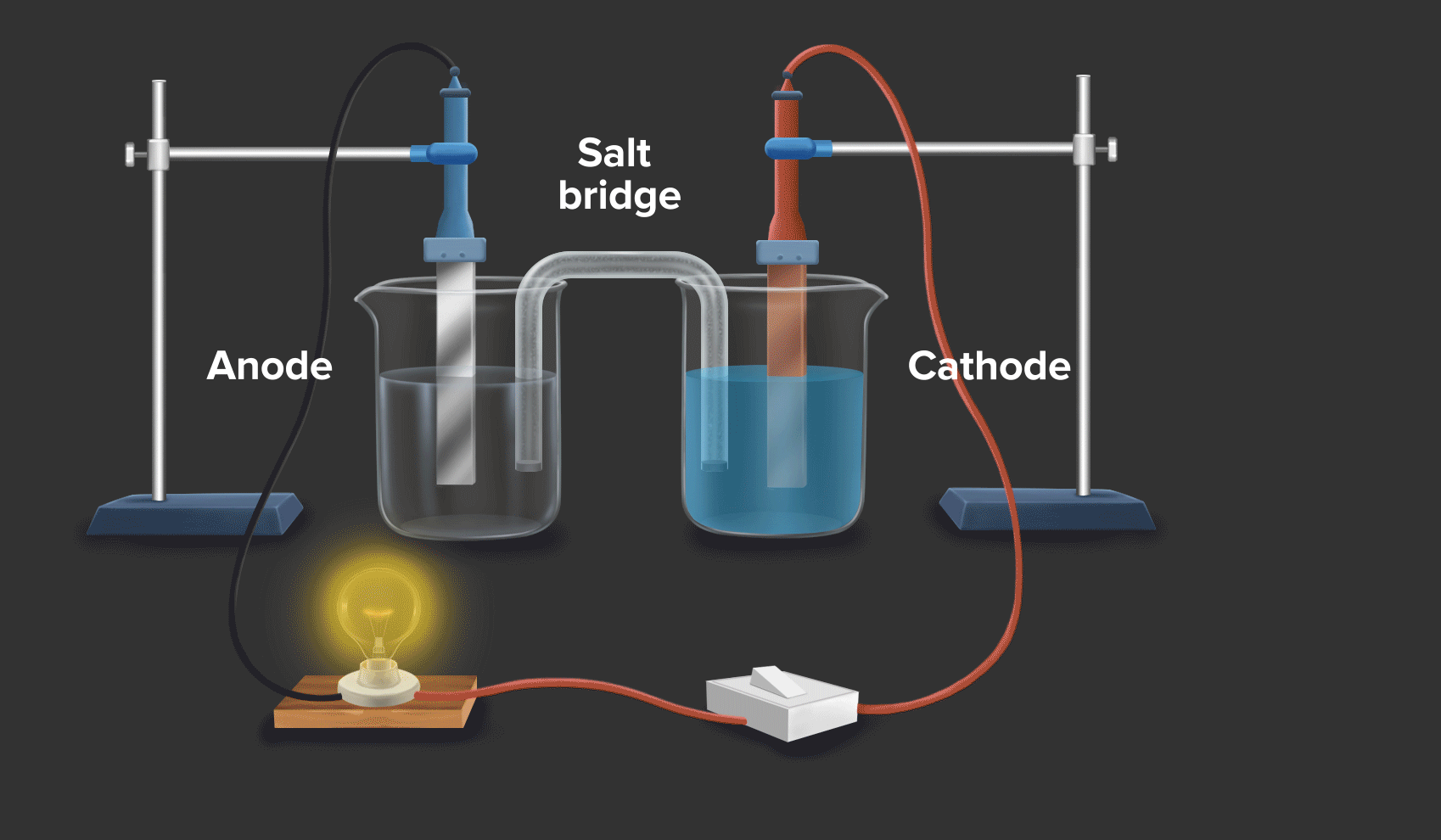
Zinc And Copper Redox . copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: Cu 2+ + 2e − → cu copper is reduced from +2 to 0. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. Reaction of zinc and copper(ii) sulfate. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. In this equation, zinc (zn) is oxidized while copper. a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. redox reactions are only spontaneous in one direction. Zn + cuso4 → znso4 + cu. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Consider the reaction of zinc metal with copper(ii) sulfate: 2zn + cu2+ → 2zn2+ + cu.
from www.aakash.ac.in
Zn + cuso4 → znso4 + cu. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. In this equation, zinc (zn) is oxidized while copper. Reaction of zinc and copper(ii) sulfate. 2zn + cu2+ → 2zn2+ + cu. redox reactions are only spontaneous in one direction. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Consider the reaction of zinc metal with copper(ii) sulfate: a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and.
Redox Reactions Definition, Types, Applications & Uses Chemistry Aakash AESL
Zinc And Copper Redox In this equation, zinc (zn) is oxidized while copper. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. In this equation, zinc (zn) is oxidized while copper. the zinc and copper redox equation is written as follows: Cu 2+ + 2e − → cu copper is reduced from +2 to 0. redox reactions are only spontaneous in one direction. Zn + cuso4 → znso4 + cu. 2zn + cu2+ → 2zn2+ + cu. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. By observing this reaction and its products,. Consider the reaction of zinc metal with copper(ii) sulfate: a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs:
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs The Art of Science Zinc And Copper Redox Consider the reaction of zinc metal with copper(ii) sulfate: Zn + cuso4 → znso4 + cu. By observing this reaction and its products,. the zinc and copper redox equation is written as follows: In this equation, zinc (zn) is oxidized while copper. redox reactions are only spontaneous in one direction. copper(ii) oxide and zinc metal react together. Zinc And Copper Redox.
From www.learner.org
A Redox Reaction Using Copper and Zinc (animation) Annenberg Learner Zinc And Copper Redox If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. the zinc and copper redox equation is written as follows: 2zn + cu2+ → 2zn2+ + cu. In this equation,. Zinc And Copper Redox.
From www.nagwa.com
Question Video Describing How Oxidation Changes a Chemical Species in the Reaction between Zinc And Copper Redox Zn + cuso4 → znso4 + cu. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. By observing this reaction and its products,. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. copper(ii) oxide. Zinc And Copper Redox.
From www.pastpapersinside.com
Redox Reaction Past Papers Inside Zinc And Copper Redox the zinc and copper redox equation is written as follows: Cu 2+ + 2e − → cu copper is reduced from +2 to 0. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. By observing this reaction and its products,. redox reactions are only spontaneous in. Zinc And Copper Redox.
From byjus.com
14.During indirect redox reaction why there is a release of zinc ions from zinc rod and not of Zinc And Copper Redox redox reactions are only spontaneous in one direction. By observing this reaction and its products,. The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. a redox reaction using copper and zinc (animation) in this demonstration, dissolved. Zinc And Copper Redox.
From www.slideshare.net
Redox= quiz part 1 with answers Zinc And Copper Redox Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Cu 2+ + 2e − → cu copper is reduced from +2 to 0. The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. 2zn + cu2+ → 2zn2+ + cu. the zinc and copper redox equation is. Zinc And Copper Redox.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc And Copper Redox redox reactions are only spontaneous in one direction. Reaction of zinc and copper(ii) sulfate. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Zn + cuso4 → znso4 + cu. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. By observing this reaction and its. Zinc And Copper Redox.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs The Art of Science Zinc And Copper Redox the zinc and copper redox equation is written as follows: Consider the reaction of zinc metal with copper(ii) sulfate: Reaction of zinc and copper(ii) sulfate. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. redox reactions are only spontaneous in one direction. Zn + cuso4. Zinc And Copper Redox.
From www.peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Zinc And Copper Redox copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. In this equation, zinc (zn) is oxidized while copper. Zn + cuso4 → znso4 + cu. 2zn + cu2+ → 2zn2+ + cu. the zinc and copper. Zinc And Copper Redox.
From www.aakash.ac.in
Redox Reactions Definition, Types, Applications & Uses Chemistry Aakash AESL Zinc And Copper Redox When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. redox reactions are only spontaneous in one direction. the zinc and copper redox equation is written as follows: In this equation, zinc (zn) is oxidized while copper. a. Zinc And Copper Redox.
From www.researchgate.net
Transition metals (iron and copper) redox recycling and its relevance... Download Scientific Zinc And Copper Redox In this equation, zinc (zn) is oxidized while copper. Reaction of zinc and copper(ii) sulfate. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Consider the reaction of zinc metal with copper(ii) sulfate: If left in the solution for a longer period of time, the zinc will gradually decay due to. Zinc And Copper Redox.
From purewaterguys.com
Crystal Quest Zinc/Copper Redox Alloy Blend, per pound Zinc And Copper Redox when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: Consider the reaction of zinc metal with copper(ii) sulfate: 2zn + cu2+ → 2zn2+ + cu. In this equation, zinc (zn) is oxidized while copper. When a strip of zinc metal is placed into a blue solution. Zinc And Copper Redox.
From fphoto.photoshelter.com
science chemistry redox reaction copper zinc Fundamental Photographs The Art of Science Zinc And Copper Redox In this equation, zinc (zn) is oxidized while copper. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: Consider the reaction of zinc metal with copper(ii) sulfate: a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with. Zinc And Copper Redox.
From www.researchgate.net
(PDF) Zinc and Copper Isotopes as Tracers of Redox Processes Zinc And Copper Redox The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zn + cuso4 → znso4 + cu. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce. Zinc And Copper Redox.
From blog.thepipingmart.com
Comparing the Reactivity of Copper vs Zinc Zinc And Copper Redox By observing this reaction and its products,. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. In this equation, zinc (zn) is oxidized while copper. When a strip of zinc. Zinc And Copper Redox.
From www.slideshare.net
Redox= quiz part 1 with answers Zinc And Copper Redox 2zn + cu2+ → 2zn2+ + cu. the zinc and copper redox equation is written as follows: Zn + cuso4 → znso4 + cu. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. By observing this reaction and its products,. Zn → zn 2+ + 2e −. Zinc And Copper Redox.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Zinc And Copper Redox a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. By observing this reaction and its products,. 2zn + cu2+ → 2zn2+ + cu. Zn + cuso4 → znso4 + cu.. Zinc And Copper Redox.
From www.learner.org
A Redox Reaction Using Copper and Zinc (animation) Annenberg Learner Zinc And Copper Redox when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: redox reactions are only spontaneous in one direction. Zn + cuso4 → znso4 + cu. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and.. Zinc And Copper Redox.
From www.slideserve.com
PPT Redox Reactions PowerPoint Presentation, free download ID1733600 Zinc And Copper Redox When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zn + cuso4 → znso4 + cu. redox reactions are only spontaneous in one direction. when a zinc rod is inserted into a beaker that contains an aqueous solution. Zinc And Copper Redox.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs The Art of Science Zinc And Copper Redox 2zn + cu2+ → 2zn2+ + cu. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Consider the reaction of zinc metal with copper(ii) sulfate: In this equation, zinc (zn) is oxidized. Zinc And Copper Redox.
From www.slideserve.com
PPT Ch. 21 Honors Chem. Electrochemistry PowerPoint Presentation, free download ID2281561 Zinc And Copper Redox By observing this reaction and its products,. redox reactions are only spontaneous in one direction. In this equation, zinc (zn) is oxidized while copper. Reaction of zinc and copper(ii) sulfate. Consider the reaction of zinc metal with copper(ii) sulfate: If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to. Zinc And Copper Redox.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate Chemistry Khan Academy YouTube Zinc And Copper Redox a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. 2zn + cu2+ → 2zn2+ + cu. The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced. Zinc And Copper Redox.
From www.slideshare.net
Redox= quiz part 1 with answers Zinc And Copper Redox a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Zn + cuso4 → znso4 + cu. 2zn + cu2+ → 2zn2+ + cu. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. By observing this reaction and its products,.. Zinc And Copper Redox.
From fphoto.photoshelter.com
science chemistry redox reaction zinc Fundamental Photographs The Art of Science Zinc And Copper Redox when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: Cu 2+ + 2e − → cu copper is reduced from +2 to 0. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. By observing this reaction and its products,. If left. Zinc And Copper Redox.
From www.youtube.com
Copper oxide + zinc powder redox reactions YouTube Zinc And Copper Redox copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Reaction of zinc and copper(ii) sulfate. By observing this reaction and its products,. the zinc and copper redox equation is written as follows: 2zn + cu2+ → 2zn2+ + cu. Zn + cuso4 → znso4 + cu. In this equation, zinc. Zinc And Copper Redox.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Redox (II) Oxidation and Reduction Zinc And Copper Redox Consider the reaction of zinc metal with copper(ii) sulfate: redox reactions are only spontaneous in one direction. Reaction of zinc and copper(ii) sulfate. By observing this reaction and its products,. Cu 2+ + 2e − → cu copper is reduced from +2 to 0. Zn + cuso4 → znso4 + cu. When a strip of zinc metal is placed. Zinc And Copper Redox.
From fyoowqcvt.blob.core.windows.net
Zinc And Copper Nitrate at Crystle Johnson blog Zinc And Copper Redox The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. Consider the reaction of zinc metal with copper(ii) sulfate: By observing this reaction and its products,. Reaction of zinc and copper(ii) sulfate. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. a simple redox reaction occurs when. Zinc And Copper Redox.
From www.thesciencehive.co.uk
Redox and Electrode Potentials* — the science sauce Zinc And Copper Redox copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. By observing this reaction and its products,. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate,. Zinc And Copper Redox.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs The Art of Science Zinc And Copper Redox The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins. Zinc And Copper Redox.
From www.youtube.com
Exploring What is Redox Technology Zinc Benefits and Copper Benefits YouTube Zinc And Copper Redox By observing this reaction and its products,. The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. . Zinc And Copper Redox.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation ID5231819 Zinc And Copper Redox Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: Consider the reaction of zinc metal with copper(ii) sulfate: redox reactions are only spontaneous in one direction. When a strip of zinc. Zinc And Copper Redox.
From www.youtube.com
Redox demo of the redox reaction of copper and zinc YouTube Zinc And Copper Redox By observing this reaction and its products,. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced. Zinc And Copper Redox.
From www.toppr.com
Electron Transfer Reactions Redox Reactions, Cells, Videos, Examples Zinc And Copper Redox In this equation, zinc (zn) is oxidized while copper. By observing this reaction and its products,. The zinc electrode dissolves to give \(\ce{zn^{2+}(aq)}\) ions, while \(\ce{cu^{2+}(aq)}\) ions are simultaneously reduced to metallic copper. a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Cu 2+ + 2e − → cu copper is reduced. Zinc And Copper Redox.
From blog.thepipingmart.com
Classifications of Metals Copper vs. Zinc Zinc And Copper Redox When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. the zinc and copper redox equation is written as follows: when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous. Zinc And Copper Redox.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs The Art of Science Zinc And Copper Redox when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: 2zn + cu2+ → 2zn2+ + cu. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. copper(ii) oxide and zinc metal react together in. Zinc And Copper Redox.