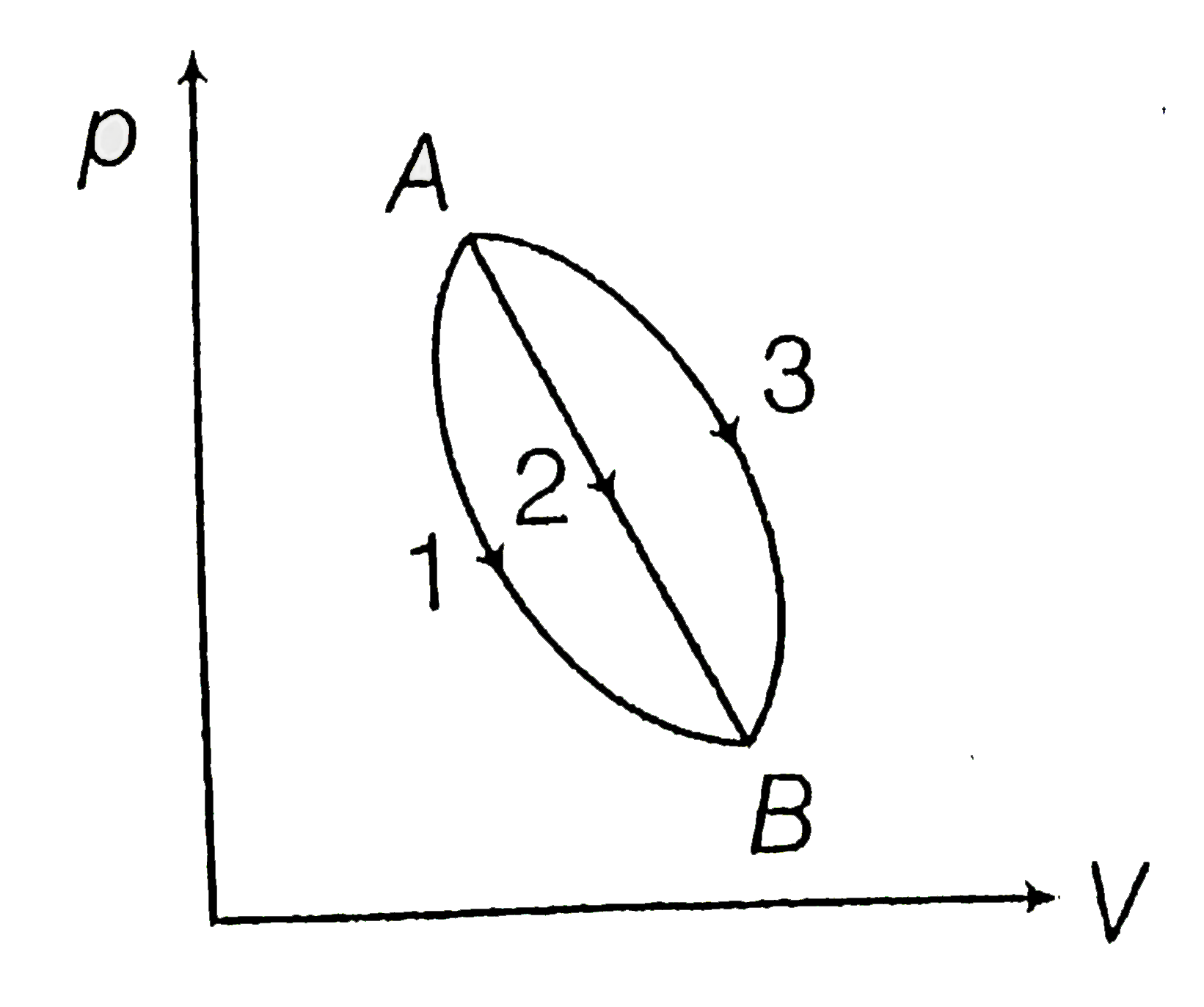
An Ideal Gas Goes From State A To State B . Absolute temperature, in k \(p\): Gas constant in kj/kgk or j/kgk; The variation of the internal energy for an ideal gas when it goes from an initial state a to a final state b is given by: Where c v is the molar heat. Volume, in m 3 \(v\): Pressure, in kpa or pa \(r\): Specific volume, in m 3 /kg \(t\): When a system is taken from the state 'a' to state 'b' along the path 'acb', it is found that a quantity of heat q = 200 j is absorbed by the system. A gas which obeys the ideal gas eos is called an ideal gas. If q 1, q 2, q 3 indicate the heat absorbed by the gas along the three processes and. $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of state back to the ideal gas law. When the particle radii are negligible and interactions forces vanish, then the. In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t is temperature of the system and δx is the infinitesimal change. The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\):
from www.doubtnut.com
In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t is temperature of the system and δx is the infinitesimal change. If q 1, q 2, q 3 indicate the heat absorbed by the gas along the three processes and. The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): A gas which obeys the ideal gas eos is called an ideal gas. Specific volume, in m 3 /kg \(t\): Absolute temperature, in k \(p\): Let δu 1,δu 2 and δu 3 be the change in the internal energy of the gas in these three processes, then: A gas undergoes a to b. When the particle radii are negligible and interactions forces vanish, then the. When a system is taken from the state 'a' to state 'b' along the path 'acb', it is found that a quantity of heat q = 200 j is absorbed by the system.
An ideal gas of mass m in a state A goes to another state B Vialpha th
An Ideal Gas Goes From State A To State B When the particle radii are negligible and interactions forces vanish, then the. Gas constant in kj/kgk or j/kgk; In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t is temperature of the system and δx is the infinitesimal change. When the particle radii are negligible and interactions forces vanish, then the. The variation of the internal energy for an ideal gas when it goes from an initial state a to a final state b is given by: The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): A gas which obeys the ideal gas eos is called an ideal gas. Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of state back to the ideal gas law. $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. A gas undergoes a to b. Specific volume, in m 3 /kg \(t\): Absolute temperature, in k \(p\): If q 1, q 2, q 3 indicate the heat absorbed by the gas along the three processes and. Where c v is the molar heat. Pressure, in kpa or pa \(r\): Let δu 1,δu 2 and δu 3 be the change in the internal energy of the gas in these three processes, then:
From www.toppr.com
One mole of an ideal gas goes from an initial state A to final state B An Ideal Gas Goes From State A To State B A gas undergoes a to b. Where c v is the molar heat. A gas which obeys the ideal gas eos is called an ideal gas. Pressure, in kpa or pa \(r\): Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of state back to the ideal gas law. The variation of the internal energy for an ideal. An Ideal Gas Goes From State A To State B.
From www.doubtnut.com
An ideal gas undergoes four different processes from the same init An Ideal Gas Goes From State A To State B A gas which obeys the ideal gas eos is called an ideal gas. Let δu 1,δu 2 and δu 3 be the change in the internal energy of the gas in these three processes, then: In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t is temperature of. An Ideal Gas Goes From State A To State B.
From mmerevise.co.uk
The Ideal Gas Equation MME An Ideal Gas Goes From State A To State B $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. A gas which obeys the ideal gas eos is called an ideal gas. Where c v is the molar heat. Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of. An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved One mole of an ideal diatomic gas goes from a to c An Ideal Gas Goes From State A To State B Pressure, in kpa or pa \(r\): A gas which obeys the ideal gas eos is called an ideal gas. Gas constant in kj/kgk or j/kgk; $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. If q 1, q 2, q 3. An Ideal Gas Goes From State A To State B.
From www.youtube.com
Two moles of a diatomic ideal gas is taken through `pT=` constant. Its An Ideal Gas Goes From State A To State B In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t is temperature of the system and δx is the infinitesimal change. The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): A gas which obeys the ideal gas eos. An Ideal Gas Goes From State A To State B.
From inspiritvr.com
Ideal Gas Law Study Guide Inspirit Learning Inc An Ideal Gas Goes From State A To State B If q 1, q 2, q 3 indicate the heat absorbed by the gas along the three processes and. Absolute temperature, in k \(p\): The variation of the internal energy for an ideal gas when it goes from an initial state a to a final state b is given by: $q$ is the heat absorbed by the gas, $w$ is. An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved The figure below is a PV diagram for an ideal gas. An Ideal Gas Goes From State A To State B Where c v is the molar heat. The variation of the internal energy for an ideal gas when it goes from an initial state a to a final state b is given by: The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): $q$ is the heat absorbed by the gas,. An Ideal Gas Goes From State A To State B.
From www.youtube.com
An ideal gas goes from state A to state B via three different processes An Ideal Gas Goes From State A To State B Where c v is the molar heat. A gas undergoes a to b. Pressure, in kpa or pa \(r\): When the particle radii are negligible and interactions forces vanish, then the. Specific volume, in m 3 /kg \(t\): Volume, in m 3 \(v\): The variation of the internal energy for an ideal gas when it goes from an initial state. An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved What is the sign of AU as the system of ideal gas An Ideal Gas Goes From State A To State B Let δu 1,δu 2 and δu 3 be the change in the internal energy of the gas in these three processes, then: When the particle radii are negligible and interactions forces vanish, then the. When a system is taken from the state 'a' to state 'b' along the path 'acb', it is found that a quantity of heat q =. An Ideal Gas Goes From State A To State B.
From askfilo.com
mple MCQ type Question [+4,−1] An ideal gas changes from state a to state.. An Ideal Gas Goes From State A To State B When a system is taken from the state 'a' to state 'b' along the path 'acb', it is found that a quantity of heat q = 200 j is absorbed by the system. Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of state back to the ideal gas law. If q 1, q 2, q 3 indicate. An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved One mole of an ideal monatomic gas undergoes a cycle An Ideal Gas Goes From State A To State B Pressure, in kpa or pa \(r\): Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of state back to the ideal gas law. $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. Volume, in m 3 \(v\): Let δu. An Ideal Gas Goes From State A To State B.
From www.tes.com
Physical Chemistry 12 The Gaseous State, Ideal Gas Law and General An Ideal Gas Goes From State A To State B The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): Where c v is the molar heat. If q 1, q 2, q 3 indicate the heat absorbed by the gas along the three processes and. A gas which obeys the ideal gas eos is called an ideal gas. Let δu. An Ideal Gas Goes From State A To State B.
From worksheets.ekocraft-appleleaf.com
The Ideal Gas Law Worksheet Worksheets For Kindergarten An Ideal Gas Goes From State A To State B The variation of the internal energy for an ideal gas when it goes from an initial state a to a final state b is given by: Pressure, in kpa or pa \(r\): Let δu 1,δu 2 and δu 3 be the change in the internal energy of the gas in these three processes, then: If q 1, q 2, q. An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved 2.00mol of a monatomic ideal gas goes from State A An Ideal Gas Goes From State A To State B Absolute temperature, in k \(p\): $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. When a system is taken from the state 'a' to state 'b' along the path 'acb', it is found that a quantity of heat q = 200. An Ideal Gas Goes From State A To State B.
From www.numerade.com
SOLVED A 1.00mol sample of an ideal monatomic gas is taken through An Ideal Gas Goes From State A To State B A gas which obeys the ideal gas eos is called an ideal gas. If q 1, q 2, q 3 indicate the heat absorbed by the gas along the three processes and. The variation of the internal energy for an ideal gas when it goes from an initial state a to a final state b is given by: Where c. An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved 2.00mol of a monatomic ideal gas goes from State A An Ideal Gas Goes From State A To State B Gas constant in kj/kgk or j/kgk; If q 1, q 2, q 3 indicate the heat absorbed by the gas along the three processes and. Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of state back to the ideal gas law. Let δu 1,δu 2 and δu 3 be the change in the internal energy of the. An Ideal Gas Goes From State A To State B.
From www.youtube.com
One mole of an ideal monoatomic gas is caused to go through the cycle An Ideal Gas Goes From State A To State B The variation of the internal energy for an ideal gas when it goes from an initial state a to a final state b is given by: Absolute temperature, in k \(p\): A gas undergoes a to b. The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): A gas which obeys. An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved 5) One mole of a monatomic ideal gas undergoes a An Ideal Gas Goes From State A To State B The variation of the internal energy for an ideal gas when it goes from an initial state a to a final state b is given by: When the particle radii are negligible and interactions forces vanish, then the. In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t. An Ideal Gas Goes From State A To State B.
From www.chegg.com
A sample of an ideal gas goes through the process An Ideal Gas Goes From State A To State B $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. Specific volume, in m 3 /kg \(t\): The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): Absolute temperature, in k \(p\): Gas. An Ideal Gas Goes From State A To State B.
From www.doubtnut.com
In the given figure an ideal gas changes its state from A to state C b An Ideal Gas Goes From State A To State B Where c v is the molar heat. Pressure, in kpa or pa \(r\): When a system is taken from the state 'a' to state 'b' along the path 'acb', it is found that a quantity of heat q = 200 j is absorbed by the system. Gas constant in kj/kgk or j/kgk; Notice that taking the limit as \(a,\;b\rightarrow 0\). An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved 1. An ideal gas in a closed system undergoes a cycle An Ideal Gas Goes From State A To State B In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t is temperature of the system and δx is the infinitesimal change. The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): Volume, in m 3 \(v\): Pressure, in kpa. An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved One mole of an ideal gas goes from state A (P= 1.2 An Ideal Gas Goes From State A To State B $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. When the particle radii are negligible and interactions forces vanish, then the. The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): A. An Ideal Gas Goes From State A To State B.
From www.youtube.com
An ideal gas goes from State A to state B via three different process An Ideal Gas Goes From State A To State B Volume, in m 3 \(v\): In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t is temperature of the system and δx is the infinitesimal change. Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of state back to the ideal gas law. Pressure, in kpa. An Ideal Gas Goes From State A To State B.
From askfilo.com
An ideal gas goes from state A (3). A monatomic gas at pressure P1 and v.. An Ideal Gas Goes From State A To State B If q 1, q 2, q 3 indicate the heat absorbed by the gas along the three processes and. When the particle radii are negligible and interactions forces vanish, then the. A gas which obeys the ideal gas eos is called an ideal gas. The simplest equation of state is the ideal gas equation of state, which is expressed as. An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved 2.00mol of a monatomic ideal gas goes from State A An Ideal Gas Goes From State A To State B Absolute temperature, in k \(p\): Where c v is the molar heat. In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t is temperature of the system and δx is the infinitesimal change. Gas constant in kj/kgk or j/kgk; Specific volume, in m 3 /kg \(t\): $q$ is. An Ideal Gas Goes From State A To State B.
From www.toppr.com
One mole of an ideal gas expanded isothermally and reversibly to half An Ideal Gas Goes From State A To State B Gas constant in kj/kgk or j/kgk; $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. When the particle radii are negligible and interactions forces vanish, then the. Where c v is the molar heat. The simplest equation of state is the. An Ideal Gas Goes From State A To State B.
From www.chegg.com
Solved 2.00mol of a monatomic ideal gas goes from State A An Ideal Gas Goes From State A To State B Volume, in m 3 \(v\): The variation of the internal energy for an ideal gas when it goes from an initial state a to a final state b is given by: A gas undergoes a to b. Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of state back to the ideal gas law. Gas constant in kj/kgk. An Ideal Gas Goes From State A To State B.
From www.britannica.com
Equation of state Definition, Ideal Gas, & Facts Britannica An Ideal Gas Goes From State A To State B Volume, in m 3 \(v\): Specific volume, in m 3 /kg \(t\): Pressure, in kpa or pa \(r\): $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. Gas constant in kj/kgk or j/kgk; Where c v is the molar heat. The. An Ideal Gas Goes From State A To State B.
From www.doubtnut.com
An ideal gas of mass m in a state A goes to another state B Vialpha th An Ideal Gas Goes From State A To State B Gas constant in kj/kgk or j/kgk; Absolute temperature, in k \(p\): A gas undergoes a to b. When the particle radii are negligible and interactions forces vanish, then the. The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): Pressure, in kpa or pa \(r\): A gas which obeys the ideal. An Ideal Gas Goes From State A To State B.
From askfilo.com
One mole of an ideal gas goes from an initial state A to final state B vi.. An Ideal Gas Goes From State A To State B Specific volume, in m 3 /kg \(t\): If q 1, q 2, q 3 indicate the heat absorbed by the gas along the three processes and. In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t is temperature of the system and δx is the infinitesimal change. Notice. An Ideal Gas Goes From State A To State B.
From askfilo.com
One mole of an ideal gas goes from an initial state A to the final state An Ideal Gas Goes From State A To State B $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. When a system is taken from the state 'a' to state 'b' along the path 'acb', it is found that a quantity of heat q = 200 j is absorbed by the. An Ideal Gas Goes From State A To State B.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET An Ideal Gas Goes From State A To State B Specific volume, in m 3 /kg \(t\): In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by tδx, where t is temperature of the system and δx is the infinitesimal change. When a system is taken from the state 'a' to state 'b' along the path 'acb', it is found that. An Ideal Gas Goes From State A To State B.
From www.toppr.com
In a cyclic process shown in the figure, an ideal gas is adiabatically An Ideal Gas Goes From State A To State B A gas undergoes a to b. Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of state back to the ideal gas law. Pressure, in kpa or pa \(r\): The variation of the internal energy for an ideal gas when it goes from an initial state a to a final state b is given by: If q 1,. An Ideal Gas Goes From State A To State B.
From www.toppr.com
A monatomic ideal gas undergoes the shown cyclic process in which the An Ideal Gas Goes From State A To State B $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. Absolute temperature, in k \(p\): Notice that taking the limit as \(a,\;b\rightarrow 0\) returns the equation of state back to the ideal gas law. A gas undergoes a to b. A gas. An Ideal Gas Goes From State A To State B.
From solvedlib.com
An ideal gas expands adiabatically as it goes from st… SolvedLib An Ideal Gas Goes From State A To State B The simplest equation of state is the ideal gas equation of state, which is expressed as \[pv=rt\] where \(m\): $q$ is the heat absorbed by the gas, $w$ is the thermodynamic work done by the gas and $\delta u$ denoted the change in the internal energy of. A gas undergoes a to b. Specific volume, in m 3 /kg \(t\):. An Ideal Gas Goes From State A To State B.