Can Standard Cell Potential Be Negative . Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The equilibrium position lies more to the left. As you can see, standard reduction potentials can be (and often are) negative. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is.
from www.slideserve.com
The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. The equilibrium position lies more to the left. As you can see, standard reduction potentials can be (and often are) negative. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur.
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free
Can Standard Cell Potential Be Negative The equilibrium position lies more to the left. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The equilibrium position lies more to the left. As you can see, standard reduction potentials can be (and often are) negative.
From www.slideserve.com
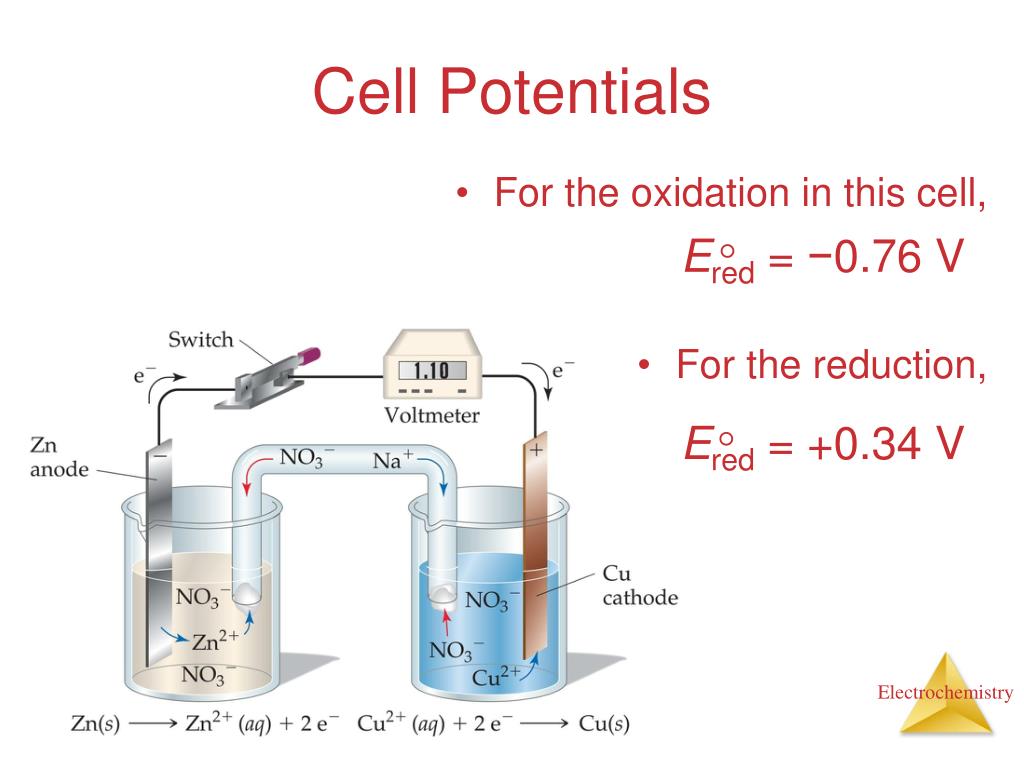
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Can Standard Cell Potential Be Negative To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard reduction potential for a metal electrode will be negative when. Can Standard Cell Potential Be Negative.
From joipgxufc.blob.core.windows.net
Standard Cell Potential Vs Cell Potential at Matthew Bryant blog Can Standard Cell Potential Be Negative The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. As you can see, standard reduction potentials can be (and often are) negative. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The standard cell. Can Standard Cell Potential Be Negative.
From dxorswgth.blob.core.windows.net
Standard Cell Potentials at Shantell Sirmans blog Can Standard Cell Potential Be Negative The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. As you can see, standard reduction potentials can be (and often are) negative. The equilibrium position lies more to the left. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed. Can Standard Cell Potential Be Negative.
From www.hanlin.com
CIE A Level Chemistry复习笔记5.3.4 Measuring the Standard Electrode Can Standard Cell Potential Be Negative Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The equilibrium position lies more to the left. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their. Can Standard Cell Potential Be Negative.
From www.pinterest.com
pe=log[e] measuved in volts.if pe is negative reduced, if pe is Can Standard Cell Potential Be Negative The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. The equilibrium position lies more to the left. The standard. Can Standard Cell Potential Be Negative.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Can Standard Cell Potential Be Negative The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The more negative (or less positive) the electrode potential, the less likely it. Can Standard Cell Potential Be Negative.
From scienceinfo.com
Cell Potential & Standard Cell Potential Can Standard Cell Potential Be Negative The equilibrium position lies more to the left. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Since the tabulated standard. Can Standard Cell Potential Be Negative.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch Can Standard Cell Potential Be Negative As you can see, standard reduction potentials can be (and often are) negative. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that. Can Standard Cell Potential Be Negative.
From dxoasuand.blob.core.windows.net
Calculate The Standard Cell Potentials Given The Following Data at Can Standard Cell Potential Be Negative As you can see, standard reduction potentials can be (and often are) negative. The equilibrium position lies more to the left. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode. Can Standard Cell Potential Be Negative.
From www.numerade.com
SOLVED 14. [20Q36,37,38] The standard Gibbs free energy (ΔG) of the Can Standard Cell Potential Be Negative Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. The equilibrium position lies more to the. Can Standard Cell Potential Be Negative.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Can Standard Cell Potential Be Negative Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The equilibrium position lies more to the left. The standard reduction potential for a metal. Can Standard Cell Potential Be Negative.
From app.pandai.org
Standard Electrode Potential Can Standard Cell Potential Be Negative The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. The equilibrium position lies more to the left.. Can Standard Cell Potential Be Negative.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Can Standard Cell Potential Be Negative The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The equilibrium position lies more to the left. The standard cell potential (\(e^o_{cell}\)) is the. Can Standard Cell Potential Be Negative.
From www.researchgate.net
EI curve showing negative halfcell potential, positive halfcell Can Standard Cell Potential Be Negative The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. As you can see, standard reduction potentials can. Can Standard Cell Potential Be Negative.
From www.numerade.com
SOLVEDkalculate the standard cell potential of the following cell at Can Standard Cell Potential Be Negative The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. To do this, chemists use the standard cell potential (e° cell), defined as the potential of. Can Standard Cell Potential Be Negative.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Can Standard Cell Potential Be Negative The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to. Can Standard Cell Potential Be Negative.
From courses.lumenlearning.com
Galvanic Cells Chemistry for Majors Can Standard Cell Potential Be Negative As you can see, standard reduction potentials can be (and often are) negative. The equilibrium position lies more to the left. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The standard reduction potential for a metal electrode will be negative when referenced to the. Can Standard Cell Potential Be Negative.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Can Standard Cell Potential Be Negative As you can see, standard reduction potentials can be (and often are) negative. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To do this, chemists use. Can Standard Cell Potential Be Negative.
From www.toppr.com
What will be standard cell potential of galvanic cell with the Can Standard Cell Potential Be Negative The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. The more negative (or less positive) the electrode. Can Standard Cell Potential Be Negative.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Can Standard Cell Potential Be Negative Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. As you can see, standard reduction potentials can be (and often are) negative.. Can Standard Cell Potential Be Negative.
From cemubadx.blob.core.windows.net
Standard Cell Potential And Standard Electrode Potential at Nina Can Standard Cell Potential Be Negative As you can see, standard reduction potentials can be (and often are) negative. The equilibrium position lies more to the left. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The more negative (or less positive) the electrode potential, the less likely it is that. Can Standard Cell Potential Be Negative.
From www.biologyonline.com
Resting potential Definition and Examples Biology Online Dictionary Can Standard Cell Potential Be Negative Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen. Can Standard Cell Potential Be Negative.
From classfullbooklice.z13.web.core.windows.net
Working Of Electrochemical Cell Can Standard Cell Potential Be Negative The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. As you can see, standard reduction potentials can be (and often are) negative. The equilibrium position lies more. Can Standard Cell Potential Be Negative.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Can Standard Cell Potential Be Negative The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. As you can see, standard reduction potentials can be (and often are) negative. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with. Can Standard Cell Potential Be Negative.
From www.chegg.com
Solved Calculate the standard cell potential for each of the Can Standard Cell Potential Be Negative To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Since the tabulated standard electrode potentials are reduction potentials, the one which. Can Standard Cell Potential Be Negative.
From navaznajeeha.blogspot.com
34+ voltaic cell potential calculator NavazNajeeha Can Standard Cell Potential Be Negative The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. As you can see, standard reduction potentials can be (and often are) negative. The equilibrium position lies more to the left. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will. Can Standard Cell Potential Be Negative.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Can Standard Cell Potential Be Negative The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. As you can see, standard reduction potentials can be (and often are) negative. The standard. Can Standard Cell Potential Be Negative.
From www.youtube.com
19.1 Calculating cell potential (HL) YouTube Can Standard Cell Potential Be Negative Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. As you can see, standard reduction potentials can be (and often are) negative. The equilibrium position lies more to the left. The standard reduction potential for a metal electrode will be negative when referenced to the. Can Standard Cell Potential Be Negative.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Can Standard Cell Potential Be Negative To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with all species in their standard. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The equilibrium position lies more to the left. Since the tabulated standard. Can Standard Cell Potential Be Negative.
From www.geeksforgeeks.org
What is Cell Potential Definition, Formula, and Examples Can Standard Cell Potential Be Negative The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which. Can Standard Cell Potential Be Negative.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Can Standard Cell Potential Be Negative The equilibrium position lies more to the left. As you can see, standard reduction potentials can be (and often are) negative. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage. Can Standard Cell Potential Be Negative.
From www.slideserve.com
PPT Calculating the Cell Potential PowerPoint Presentation, free Can Standard Cell Potential Be Negative The equilibrium position lies more to the left. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Since the tabulated standard electrode potentials are reduction potentials, the one which. Can Standard Cell Potential Be Negative.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Can Standard Cell Potential Be Negative Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. The equilibrium position lies more to the left. To do this, chemists use. Can Standard Cell Potential Be Negative.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Can Standard Cell Potential Be Negative As you can see, standard reduction potentials can be (and often are) negative. The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get.. Can Standard Cell Potential Be Negative.
From www.chegg.com
Solved Calculate the standard cell potential of the Can Standard Cell Potential Be Negative The standard reduction potential for a metal electrode will be negative when referenced to the standard hydrogen electrode if the metal ion is. As you can see, standard reduction potentials can be (and often are) negative. To do this, chemists use the standard cell potential (e° cell), defined as the potential of a cell measured under standard conditions—that is, with. Can Standard Cell Potential Be Negative.