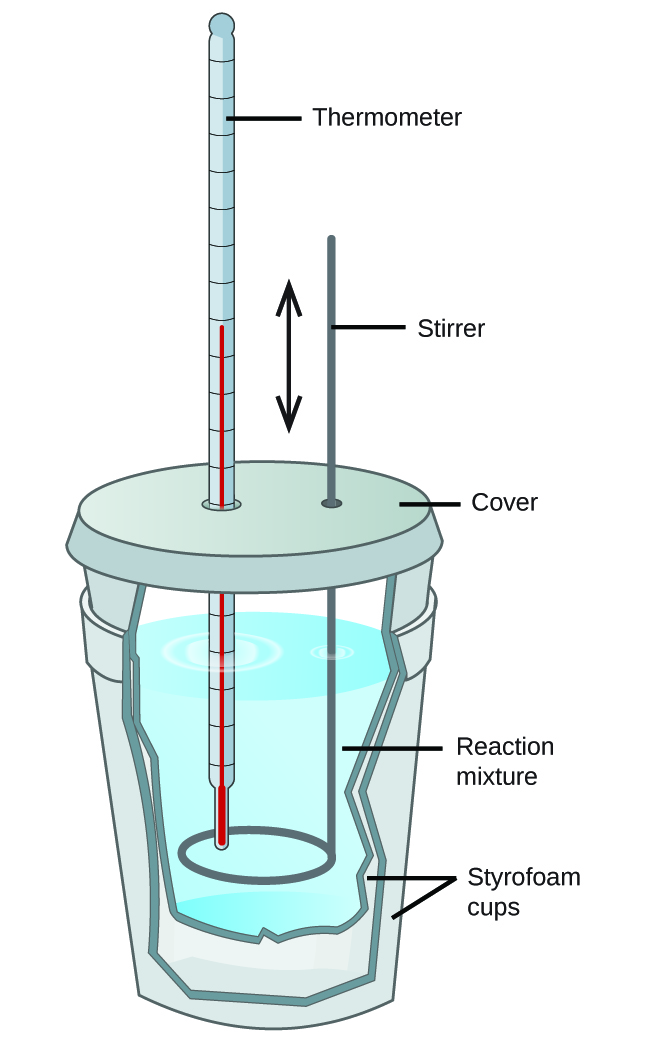
Calorimeter Experiment Calculation . Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. measuring heat transfers is called calorimetry. the calorimetry calculator can help you solve complex calorimetry problems. The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Calculate and interpret heat and related properties using typical calorimetry data explain the technique of calorimetry; We have calculated q, the energy given off to the surroundings (the enthalpy change). apply the first law of thermodynamics to calorimetry. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. The units of q are joules. It can analyze the heat exchange between up to 3 objects.
from wisc.pb.unizin.org
the calorimetry calculator can help you solve complex calorimetry problems. explain the technique of calorimetry; Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. Calculate and interpret heat and related properties using typical calorimetry data The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. apply the first law of thermodynamics to calorimetry. It can analyze the heat exchange between up to 3 objects. measuring heat transfers is called calorimetry. The units of q are joules.
5.2 Calorimetry Chemistry
Calorimeter Experiment Calculation the calorimetry calculator can help you solve complex calorimetry problems. We have calculated q, the energy given off to the surroundings (the enthalpy change). Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It can analyze the heat exchange between up to 3 objects. the calorimetry calculator can help you solve complex calorimetry problems. Calculate and interpret heat and related properties using typical calorimetry data The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. measuring heat transfers is called calorimetry. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. explain the technique of calorimetry; apply the first law of thermodynamics to calorimetry. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. The units of q are joules.
From dxoblibct.blob.core.windows.net
Calorimetry Calculations Examples at Miriam Matos blog Calorimeter Experiment Calculation Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The units of q are joules. the calorimetry calculator can help you solve complex calorimetry problems. Calculate and interpret heat and related properties using typical calorimetry data It can analyze the heat exchange between up to 3 objects. We have calculated q, the. Calorimeter Experiment Calculation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Experiment Calculation apply the first law of thermodynamics to calorimetry. the calorimetry calculator can help you solve complex calorimetry problems. measuring heat transfers is called calorimetry. The units of q are joules. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It can analyze the heat exchange between up to 3 objects.. Calorimeter Experiment Calculation.
From dxopjumlc.blob.core.windows.net
Calorimeter Calibration Calculator at Jeffery Williams blog Calorimeter Experiment Calculation experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. We have calculated q, the energy given off to the surroundings (the enthalpy change). the calorimetry calculator can help you solve complex calorimetry problems. Calculate and interpret heat and related properties using typical. Calorimeter Experiment Calculation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter Experiment Calculation Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. It can analyze the heat exchange between up to 3 objects. explain the technique of calorimetry; in this experiment you will heat a known mass of a metal to a known temperature and. Calorimeter Experiment Calculation.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimeter Experiment Calculation apply the first law of thermodynamics to calorimetry. the calorimetry calculator can help you solve complex calorimetry problems. The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter. Calorimeter Experiment Calculation.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Experiment Calculation apply the first law of thermodynamics to calorimetry. The units of q are joules. It can analyze the heat exchange between up to 3 objects. The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into. Calorimeter Experiment Calculation.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimeter Experiment Calculation apply the first law of thermodynamics to calorimetry. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. explain the technique. Calorimeter Experiment Calculation.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimeter Experiment Calculation The units of q are joules. measuring heat transfers is called calorimetry. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The diagram shows a simple. Calorimeter Experiment Calculation.
From learningcampusfox.z1.web.core.windows.net
Coffee Cup Calorimetry Lab Report Calorimeter Experiment Calculation apply the first law of thermodynamics to calorimetry. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The units of q are joules. measuring heat transfers. Calorimeter Experiment Calculation.
From cerifsbe.blob.core.windows.net
Calorimetry Fuels Experiment at Francis Edler blog Calorimeter Experiment Calculation Calculate and interpret heat and related properties using typical calorimetry data apply the first law of thermodynamics to calorimetry. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. measuring heat transfers is called calorimetry. The units of q are joules. experiment. Calorimeter Experiment Calculation.
From studylib.net
Calorimetry and Coffee Cups Calorimeter Experiment Calculation The units of q are joules. Calculate and interpret heat and related properties using typical calorimetry data We have calculated q, the energy given off to the surroundings (the enthalpy change). The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. It can analyze the heat exchange between up to 3 objects. experiment. Calorimeter Experiment Calculation.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimeter Experiment Calculation The units of q are joules. apply the first law of thermodynamics to calorimetry. The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. explain the technique of calorimetry; the calorimetry calculator can help you solve complex calorimetry problems. in this experiment you will heat a known mass of a. Calorimeter Experiment Calculation.
From studylib.net
Bomb Calorimeter Lab Sheet Calorimeter Experiment Calculation It can analyze the heat exchange between up to 3 objects. Calculate and interpret heat and related properties using typical calorimetry data The units of q are joules. apply the first law of thermodynamics to calorimetry. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Calorimeter Experiment Calculation.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Experiment Calculation We have calculated q, the energy given off to the surroundings (the enthalpy change). in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It can analyze the heat. Calorimeter Experiment Calculation.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimeter Experiment Calculation The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. apply the first law of thermodynamics to calorimetry. measuring heat transfers is called calorimetry. explain the technique of calorimetry; Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. the calorimetry calculator can help. Calorimeter Experiment Calculation.
From www.chegg.com
Solved Combustion (bomb) calorimeter. In an experiment, a Calorimeter Experiment Calculation The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. Calculate and interpret heat and related properties using typical calorimetry data the calorimetry calculator can help you solve complex calorimetry problems. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold. Calorimeter Experiment Calculation.
From classlibrarycordeiro.z21.web.core.windows.net
How To Calculate Calorimetry Problems Calorimeter Experiment Calculation apply the first law of thermodynamics to calorimetry. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. explain the technique of calorimetry; Calculate and interpret heat and related properties using typical calorimetry data to make sure you get accurate results. Calorimeter Experiment Calculation.
From www.chegg.com
Solved Part A Below is a calorimetry experiment. Calculate Calorimeter Experiment Calculation experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. the calorimetry calculator can help you solve complex calorimetry problems. explain the technique of calorimetry; . Calorimeter Experiment Calculation.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimeter Experiment Calculation The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The units of q are joules. explain the technique of calorimetry; measuring heat transfers is called calorimetry. It can analyze the heat exchange between up to. Calorimeter Experiment Calculation.
From answermagicmatney.z21.web.core.windows.net
Coffee Cup Calorimetry Experiment Calorimeter Experiment Calculation apply the first law of thermodynamics to calorimetry. The units of q are joules. measuring heat transfers is called calorimetry. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. the calorimetry calculator can help you solve complex calorimetry problems. . Calorimeter Experiment Calculation.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter Calorimeter Experiment Calculation We have calculated q, the energy given off to the surroundings (the enthalpy change). in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. It can analyze the heat exchange between up to 3 objects. Calculate and interpret heat and related properties using typical calorimetry. Calorimeter Experiment Calculation.
From ceglnfrl.blob.core.windows.net
Calorimeter Physics at Daniel Armstrong blog Calorimeter Experiment Calculation We have calculated q, the energy given off to the surroundings (the enthalpy change). apply the first law of thermodynamics to calorimetry. Calculate and interpret heat and related properties using typical calorimetry data The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. Compare heat flow from hot to cold objects in an. Calorimeter Experiment Calculation.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimeter Experiment Calculation Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. explain the technique of calorimetry; apply the first law of thermodynamics to calorimetry. measuring heat transfers is called calorimetry. It can analyze the heat exchange between up to 3 objects. experiment 6 ∙ calorimetry 6‐3 consider what happens when a. Calorimeter Experiment Calculation.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube Calorimeter Experiment Calculation The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. It can analyze the heat exchange between up to 3 objects. We have calculated q, the energy given off to the surroundings (the. Calorimeter Experiment Calculation.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Experiment Calculation to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. apply the first law of thermodynamics to calorimetry. measuring heat transfers is called calorimetry. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water. Calorimeter Experiment Calculation.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter Experiment Calculation apply the first law of thermodynamics to calorimetry. We have calculated q, the energy given off to the surroundings (the enthalpy change). Calculate and interpret heat and related properties using typical calorimetry data explain the technique of calorimetry; The units of q are joules. Compare heat flow from hot to cold objects in an ideal calorimeter versus a. Calorimeter Experiment Calculation.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimeter Experiment Calculation We have calculated q, the energy given off to the surroundings (the enthalpy change). to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.. Calorimeter Experiment Calculation.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Calorimeter Experiment Calculation apply the first law of thermodynamics to calorimetry. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. It can analyze the heat exchange between up to 3 objects. We have calculated q, the energy given off to the surroundings (the enthalpy change).. Calorimeter Experiment Calculation.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Experiment Calculation to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Calculate and interpret heat and related properties using typical calorimetry data The units of. Calorimeter Experiment Calculation.
From www.studypool.com
SOLUTION calorimetry enthalpy change calculation Studypool Calorimeter Experiment Calculation It can analyze the heat exchange between up to 3 objects. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. in this experiment you will heat a known mass of a metal to a known temperature. Calorimeter Experiment Calculation.
From cechiggi.blob.core.windows.net
Lab Calorimetry And Specific Heat at Alma Smith blog Calorimeter Experiment Calculation We have calculated q, the energy given off to the surroundings (the enthalpy change). measuring heat transfers is called calorimetry. apply the first law of thermodynamics to calorimetry. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. Compare heat flow from hot to cold objects in. Calorimeter Experiment Calculation.
From exohkbfgq.blob.core.windows.net
Calorimeter For Experiment at Lillian Bordner blog Calorimeter Experiment Calculation to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. measuring heat transfers is called calorimetry. explain the technique of calorimetry; in this experiment you will heat a known mass. Calorimeter Experiment Calculation.
From www.researchgate.net
Temperature measurements during a typical calorimetry experiment Calorimeter Experiment Calculation measuring heat transfers is called calorimetry. explain the technique of calorimetry; We have calculated q, the energy given off to the surroundings (the enthalpy change). Calculate and interpret heat and related properties using typical calorimetry data experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold. Calorimeter Experiment Calculation.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimeter Experiment Calculation apply the first law of thermodynamics to calorimetry. We have calculated q, the energy given off to the surroundings (the enthalpy change). It can analyze the heat exchange between up to 3 objects. Calculate and interpret heat and related properties using typical calorimetry data to make sure you get accurate results you need to calculate the calorimeter constant,. Calorimeter Experiment Calculation.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimeter Experiment Calculation The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. measuring heat transfers is called calorimetry. Calculate and interpret heat and related properties using typical calorimetry data The units of q are joules. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat. Calorimeter Experiment Calculation.