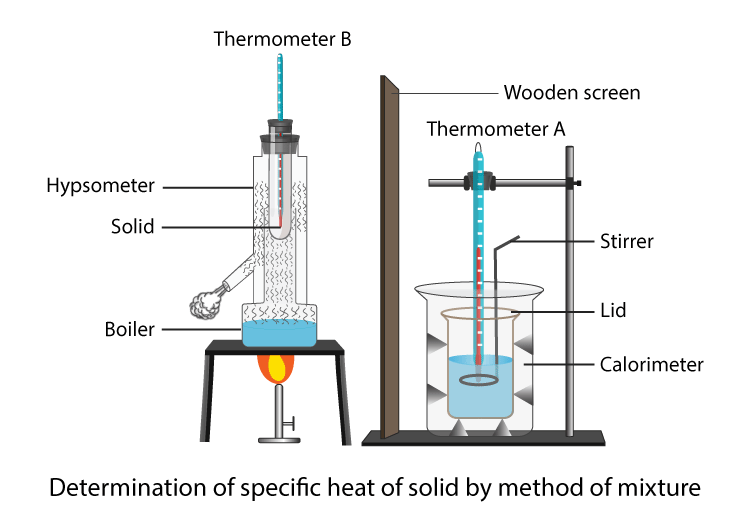
Calorimeter Specific Heat Capacity Of Water . this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. T2 is the final temperature, and t1 is the starting temperature. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. Specific heat is the amount of. the actual value for the specific heat capacity of water is 4,200 j/kg°c. The calculated value does not match exactly. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. calculate the specific heat capacity of water, using equation 2 from the introduction. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal.
from cechiggi.blob.core.windows.net
normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. T2 is the final temperature, and t1 is the starting temperature. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). Specific heat is the amount of. The calculated value does not match exactly. the calorimetry calculator can help you solve complex calorimetry problems. the actual value for the specific heat capacity of water is 4,200 j/kg°c. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample.
Lab Calorimetry And Specific Heat at Alma Smith blog
Calorimeter Specific Heat Capacity Of Water the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. T2 is the final temperature, and t1 is the starting temperature. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. the actual value for the specific heat capacity of water is 4,200 j/kg°c. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. Specific heat is the amount of. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. calculate the specific heat capacity of water, using equation 2 from the introduction. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. The calculated value does not match exactly.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Specific Heat Capacity Of Water Specific heat is the amount of. this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p).. Calorimeter Specific Heat Capacity Of Water.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Calorimeter Specific Heat Capacity Of Water the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. calculate the specific heat capacity of water, using equation 2 from the introduction. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184. Calorimeter Specific Heat Capacity Of Water.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Specific Heat Capacity Of Water the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). Specific heat is the amount of. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal. Calorimeter Specific Heat Capacity Of Water.
From slidetodoc.com
CHEM 1011 Calorimetry The Determination of the Specific Calorimeter Specific Heat Capacity Of Water T2 is the final temperature, and t1 is the starting temperature. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). calculate the specific heat capacity of water, using equation 2 from the introduction. . Calorimeter Specific Heat Capacity Of Water.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimeter Specific Heat Capacity Of Water normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. Specific heat is the amount of. the actual value for the specific heat capacity of water is 4,200 j/kg°c. T2 is the final temperature, and t1 is the starting temperature. . Calorimeter Specific Heat Capacity Of Water.
From users.highland.edu
Calorimetry Calorimeter Specific Heat Capacity Of Water T2 is the final temperature, and t1 is the starting temperature. the actual value for the specific heat capacity of water is 4,200 j/kg°c. Specific heat is the amount of. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. calculate the specific. Calorimeter Specific Heat Capacity Of Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Specific Heat Capacity Of Water calculate the specific heat capacity of water, using equation 2 from the introduction. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). the calorimetry calculator can help you solve complex calorimetry problems. Specific. Calorimeter Specific Heat Capacity Of Water.
From lessoncampuswildlife.z22.web.core.windows.net
What Is Used To Measure Heat Calorimeter Specific Heat Capacity Of Water the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. the calorimetry calculator can help you solve complex. Calorimeter Specific Heat Capacity Of Water.
From www.numerade.com
SOLVED 'A 89.2 g piece of aluminum (which has molar heat capacity of Calorimeter Specific Heat Capacity Of Water the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. Specific heat is the amount of. the calorimetry calculator can help you solve complex. Calorimeter Specific Heat Capacity Of Water.
From cechiggi.blob.core.windows.net
Lab Calorimetry And Specific Heat at Alma Smith blog Calorimeter Specific Heat Capacity Of Water the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. It can analyze the heat exchange between up to 3 objects. T2 is the final temperature, and t1 is the starting temperature. the specific heat (c s) of a substance is the amount of. Calorimeter Specific Heat Capacity Of Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Specific Heat Capacity Of Water this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. the actual value for the specific heat capacity of water is 4,200 j/kg°c. It can analyze the heat exchange between up to 3 objects. T2 is the final temperature, and t1 is the starting temperature. The calculated value does. Calorimeter Specific Heat Capacity Of Water.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Specific Heat Capacity Of Water normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. It can analyze the heat exchange between up to 3 objects. the actual value for the specific heat capacity of water is 4,200 j/kg°c. Specific heat is the amount of. . Calorimeter Specific Heat Capacity Of Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Specific Heat Capacity Of Water the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. the specific heat (c s) of a substance. Calorimeter Specific Heat Capacity Of Water.
From exouxbqmv.blob.core.windows.net
Calorimetry Heat Of Formation at Dolores Slama blog Calorimeter Specific Heat Capacity Of Water the calorimetry calculator can help you solve complex calorimetry problems. The calculated value does not match exactly. calculate the specific heat capacity of water, using equation 2 from the introduction. the actual value for the specific heat capacity of water is 4,200 j/kg°c. T2 is the final temperature, and t1 is the starting temperature. the specific. Calorimeter Specific Heat Capacity Of Water.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimeter Specific Heat Capacity Of Water the actual value for the specific heat capacity of water is 4,200 j/kg°c. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. the calorimetry calculator can help you solve complex calorimetry problems. normally it can be done by heating a piece. Calorimeter Specific Heat Capacity Of Water.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimeter Specific Heat Capacity Of Water Specific heat is the amount of. this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. the actual value for the specific heat capacity of water is 4,200 j/kg°c. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of. Calorimeter Specific Heat Capacity Of Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Specific Heat Capacity Of Water normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. the actual value for the specific heat capacity of water is 4,200 j/kg°c. Specific heat is the amount of. the specific heat (c s) of a substance is the amount. Calorimeter Specific Heat Capacity Of Water.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Calorimeter Specific Heat Capacity Of Water calculate the specific heat capacity of water, using equation 2 from the introduction. the calorimetry calculator can help you solve complex calorimetry problems. The calculated value does not match exactly. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. Specific heat. Calorimeter Specific Heat Capacity Of Water.
From cewwmzhd.blob.core.windows.net
What Is The Heat Capacity Of The Calorimeter Due To at Peter Brunton blog Calorimeter Specific Heat Capacity Of Water the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. the calorimetry calculator can help you solve complex calorimetry problems. this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. It can analyze. Calorimeter Specific Heat Capacity Of Water.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimeter Specific Heat Capacity Of Water the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. Specific heat is the amount of. T2 is the final temperature, and t1 is the starting temperature. normally it can be done by heating a piece of nickel or something, recording the temperature. Calorimeter Specific Heat Capacity Of Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Specific Heat Capacity Of Water this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. the calorimetry calculator can help you solve complex calorimetry problems. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. . Calorimeter Specific Heat Capacity Of Water.
From www.sarthaks.com
Figure shows an electrical calorimeter to determine specific heat Calorimeter Specific Heat Capacity Of Water this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). calculate the specific heat capacity. Calorimeter Specific Heat Capacity Of Water.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimeter Specific Heat Capacity Of Water The calculated value does not match exactly. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. the. Calorimeter Specific Heat Capacity Of Water.
From www.askwillonline.com
Specific Heat Capacity and Latent Heat Experiments In Physics Ask Calorimeter Specific Heat Capacity Of Water the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). . Calorimeter Specific Heat Capacity Of Water.
From cechiggi.blob.core.windows.net
Lab Calorimetry And Specific Heat at Alma Smith blog Calorimeter Specific Heat Capacity Of Water the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). Specific heat is the amount of. the calorimetry calculator can help you solve complex calorimetry problems. the actual value for the specific heat capacity. Calorimeter Specific Heat Capacity Of Water.
From cejcuygf.blob.core.windows.net
How To Find Specific Heat Capacity Of Calorimeter at Judy Cifuentes blog Calorimeter Specific Heat Capacity Of Water the calorimetry calculator can help you solve complex calorimetry problems. normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. The calculated value does not match exactly. T2 is the final temperature, and t1 is the starting temperature. the specific. Calorimeter Specific Heat Capacity Of Water.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter Calorimeter Specific Heat Capacity Of Water T2 is the final temperature, and t1 is the starting temperature. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. The calculated value does not match exactly. It can analyze the heat exchange between up to 3 objects. the actual value for the. Calorimeter Specific Heat Capacity Of Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Specific Heat Capacity Of Water the actual value for the specific heat capacity of water is 4,200 j/kg°c. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1 °c requires 4.184 j. this specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. . Calorimeter Specific Heat Capacity Of Water.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Calorimeter Specific Heat Capacity Of Water Specific heat is the amount of. The calculated value does not match exactly. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. T2 is the final temperature, and t1 is the starting temperature. the calorimetry calculator can help you solve complex calorimetry. Calorimeter Specific Heat Capacity Of Water.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer Calorimeter Specific Heat Capacity Of Water the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by.. Calorimeter Specific Heat Capacity Of Water.
From alevelphysicsnotes.com
Mr Toogood Physics Specific heat capacity Calorimeter Specific Heat Capacity Of Water calculate the specific heat capacity of water, using equation 2 from the introduction. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. Specific heat is the amount of. The calculated value does not match exactly. this specific heat calculator is a. Calorimeter Specific Heat Capacity Of Water.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimeter Specific Heat Capacity Of Water the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by. The calculated value does not match exactly. Specific heat is the amount of. the specific heat of water is 4.184 j/g °c (table \(\pageindex{1}\)), so to heat 1 g of water by 1. Calorimeter Specific Heat Capacity Of Water.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Calorimeter Specific Heat Capacity Of Water the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). Specific heat is the amount of. calculate the specific heat capacity of water, using equation 2 from the introduction. The calculated value does not match. Calorimeter Specific Heat Capacity Of Water.
From ceonxwqk.blob.core.windows.net
Block Diagram Of Calorimeter at Rachell Anderson blog Calorimeter Specific Heat Capacity Of Water normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. the actual value for the specific heat capacity of water is 4,200 j/kg°c. the specific heat (c s) of a substance is the amount of energy needed to raise the. Calorimeter Specific Heat Capacity Of Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter Specific Heat Capacity Of Water It can analyze the heat exchange between up to 3 objects. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). calculate the specific heat capacity of water, using equation 2 from the introduction. Specific. Calorimeter Specific Heat Capacity Of Water.