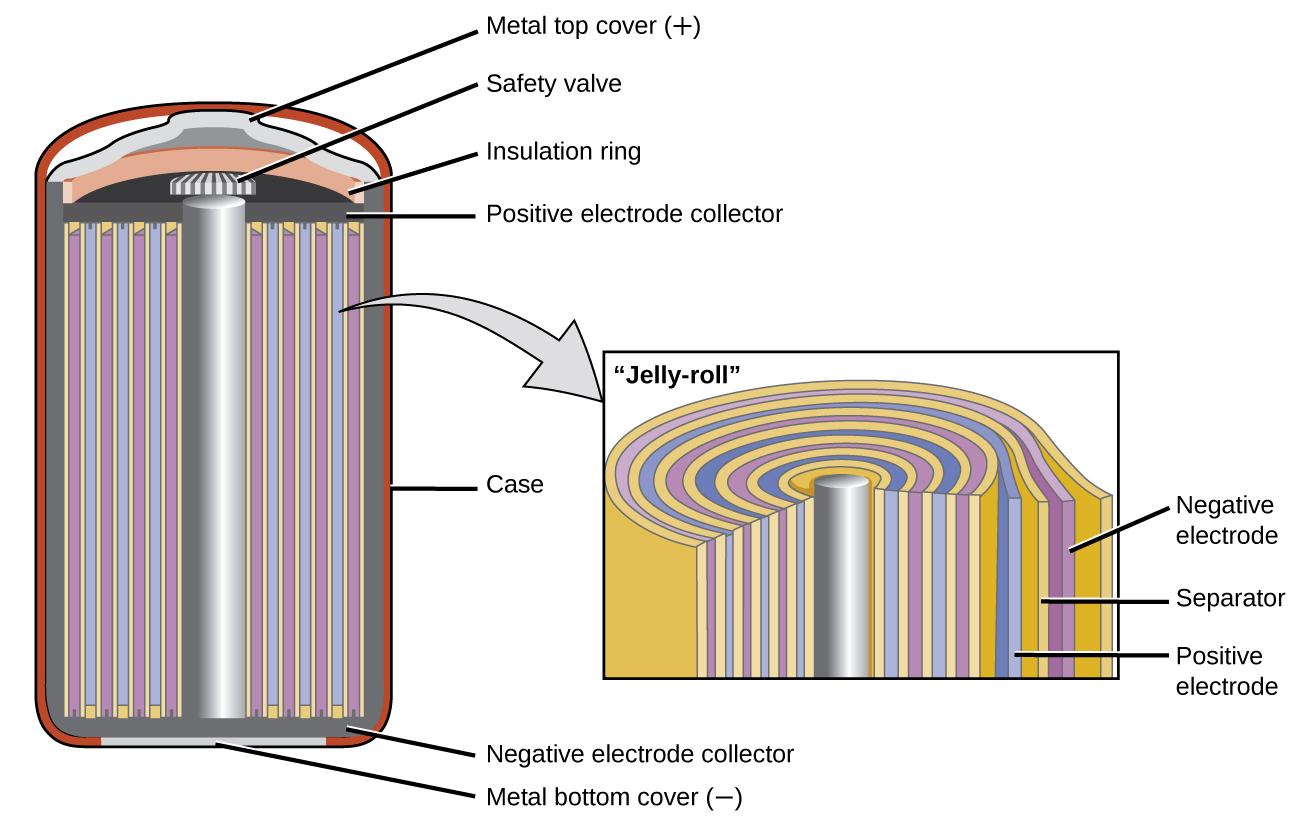
How Can A 6V Battery Be Made From Alkaline Cells . Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. , which has a higher voltage than a. Potassium or sodium hydroxide is the alkaline. A number of cells can be connected in series to make a. Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. Batteries provide electrical energy from chemical energy. In this video we’ll look at how alkaline based batteries are made. All of the common disposable alkaline batteries from aaa to d are single cells. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. The exception is the 9v battery which actually has six smaller cells inside of it. The process starts with a one piece metal case which. A simple cell can be made by connecting two different metals in contact with an electrolyte. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts.
from chem.libretexts.org
, which has a higher voltage than a. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts. The process starts with a one piece metal case which. The exception is the 9v battery which actually has six smaller cells inside of it. A simple cell can be made by connecting two different metals in contact with an electrolyte. In this video we’ll look at how alkaline based batteries are made. Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. Potassium or sodium hydroxide is the alkaline. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. Batteries provide electrical energy from chemical energy.
6.7 Batteries and Fuel Cells Chemistry LibreTexts
How Can A 6V Battery Be Made From Alkaline Cells Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. Potassium or sodium hydroxide is the alkaline. Batteries provide electrical energy from chemical energy. A number of cells can be connected in series to make a. Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. In this video we’ll look at how alkaline based batteries are made. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts. Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. The process starts with a one piece metal case which. A simple cell can be made by connecting two different metals in contact with an electrolyte. All of the common disposable alkaline batteries from aaa to d are single cells. The exception is the 9v battery which actually has six smaller cells inside of it. , which has a higher voltage than a.
From ar.inspiredpencil.com
Alkaline Battery Diagram How Can A 6V Battery Be Made From Alkaline Cells In this video we’ll look at how alkaline based batteries are made. Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. Potassium or sodium hydroxide is the alkaline. All of the common disposable alkaline batteries from aaa to d are single cells. The exception is the 9v battery which actually has six. How Can A 6V Battery Be Made From Alkaline Cells.
From batteryguy.com
What are Alkaline Batteries? Knowledge Base How Can A 6V Battery Be Made From Alkaline Cells Batteries provide electrical energy from chemical energy. Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. , which has a higher voltage than a. In this video we’ll look at how alkaline based batteries are made. A number of cells can be connected in series to make a. Explore the essential elements. How Can A 6V Battery Be Made From Alkaline Cells.
From www.vlad.fr
Battery alkaline 6V 11A GP Vlad How Can A 6V Battery Be Made From Alkaline Cells Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. All of the common disposable alkaline batteries from aaa to d are single cells. The process starts with a one piece metal case which. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. Potassium or sodium hydroxide is the. How Can A 6V Battery Be Made From Alkaline Cells.
From www.slideserve.com
PPT Harnessing the Power of Voltaic Cells Batteries and Corrosion How Can A 6V Battery Be Made From Alkaline Cells Batteries provide electrical energy from chemical energy. A number of cells can be connected in series to make a. The process starts with a one piece metal case which. All of the common disposable alkaline batteries from aaa to d are single cells. , which has a higher voltage than a. Potassium or sodium hydroxide is the alkaline. Alkaline batteries. How Can A 6V Battery Be Made From Alkaline Cells.
From www.slideserve.com
PPT Chemistry 100 Chapter 20 PowerPoint Presentation, free download How Can A 6V Battery Be Made From Alkaline Cells The process starts with a one piece metal case which. , which has a higher voltage than a. In this video we’ll look at how alkaline based batteries are made. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. The exception is the 9v battery which actually has six smaller cells inside of it. Thus, the. How Can A 6V Battery Be Made From Alkaline Cells.
From www.batteries4pro.com
Alkaline battery 6V 4LR25 How Can A 6V Battery Be Made From Alkaline Cells Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. All of the common disposable alkaline batteries from aaa to d are single cells. Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. , which has a higher voltage than a. The exception is. How Can A 6V Battery Be Made From Alkaline Cells.
From www.bhphotovideo.com
Exell Battery A28PX 6V Alkaline Battery A28PX B&H Photo Video How Can A 6V Battery Be Made From Alkaline Cells Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. In this video we’ll look at how alkaline based batteries are made. All of the common disposable alkaline batteries from aaa to d are single cells. , which has a higher voltage than a. Batteries provide electrical energy from chemical energy. Alkaline batteries. How Can A 6V Battery Be Made From Alkaline Cells.
From chem.libretexts.org
6.7 Batteries and Fuel Cells Chemistry LibreTexts How Can A 6V Battery Be Made From Alkaline Cells , which has a higher voltage than a. All of the common disposable alkaline batteries from aaa to d are single cells. The process starts with a one piece metal case which. In this video we’ll look at how alkaline based batteries are made. Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each. How Can A 6V Battery Be Made From Alkaline Cells.
From www.thebatterysupplier.com
SAFT LS14500 AA 3.6 Volt Lithium Batteries 2600 mAh 250 Pack How Can A 6V Battery Be Made From Alkaline Cells Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. The process starts with a one piece metal case which. In this video we’ll look at how alkaline based batteries are made. Potassium or sodium. How Can A 6V Battery Be Made From Alkaline Cells.
From www.stinehome.com
Duracell Alkaline Lantern Battery 6 volts 1 pk Stine Home + Yard How Can A 6V Battery Be Made From Alkaline Cells In this video we’ll look at how alkaline based batteries are made. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts. , which has a higher voltage than a. Thus, the chemical composition inside the battery is very. How Can A 6V Battery Be Made From Alkaline Cells.
From whisenanttere1995.blogspot.com
How Can You Make 6 Volt Batteries Easy to Connect So They Can Be How Can A 6V Battery Be Made From Alkaline Cells The process starts with a one piece metal case which. Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. In this video we’ll look at how alkaline based batteries are made. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts. All of the. How Can A 6V Battery Be Made From Alkaline Cells.
From www.aliexpress.com
12pcs/lot 4LR44 476A L1325 6V Dry Alkaline Battery Cells Car Remote How Can A 6V Battery Be Made From Alkaline Cells Batteries provide electrical energy from chemical energy. , which has a higher voltage than a. The exception is the 9v battery which actually has six smaller cells inside of it. In this video we’ll look at how alkaline based batteries are made. Potassium or sodium hydroxide is the alkaline. All of the common disposable alkaline batteries from aaa to d. How Can A 6V Battery Be Made From Alkaline Cells.
From byjus.com
How Do Alkaline Batteries Work Structure of Alkaline Battery How Can A 6V Battery Be Made From Alkaline Cells The process starts with a one piece metal case which. The exception is the 9v battery which actually has six smaller cells inside of it. Potassium or sodium hydroxide is the alkaline. Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. A number of cells can be connected in series to make. How Can A 6V Battery Be Made From Alkaline Cells.
From whisenanttere1995.blogspot.com
How Can You Make 6 Volt Batteries Easy to Connect So They Can Be How Can A 6V Battery Be Made From Alkaline Cells The exception is the 9v battery which actually has six smaller cells inside of it. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. A fully discharged alkaline cell (nominal voltage 1.5 volts) still. How Can A 6V Battery Be Made From Alkaline Cells.
From www.micropower-battery.com
How Does an Alkaline Battery Work ? Microcell Battery How Can A 6V Battery Be Made From Alkaline Cells A simple cell can be made by connecting two different metals in contact with an electrolyte. In this video we’ll look at how alkaline based batteries are made. Potassium or sodium hydroxide is the alkaline. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts. A number of cells can be connected. How Can A 6V Battery Be Made From Alkaline Cells.
From www.myfloridahomeenergy.com
Batteries for Home Electronics My Florida Home Energy How Can A 6V Battery Be Made From Alkaline Cells Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. , which has a higher voltage than a. Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. The process starts with a one piece metal case which. A fully discharged. How Can A 6V Battery Be Made From Alkaline Cells.
From schematicfixtrysted.z22.web.core.windows.net
Alkaline Cell Diagram How Can A 6V Battery Be Made From Alkaline Cells The process starts with a one piece metal case which. The exception is the 9v battery which actually has six smaller cells inside of it. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. Potassium or sodium hydroxide is the alkaline. , which has a higher voltage than a. Batteries provide electrical energy from chemical energy.. How Can A 6V Battery Be Made From Alkaline Cells.
From www.homedepot.com
Energizer MAX Alkaline 6Volt Battery, 1 Pack 5291 The Home Depot How Can A 6V Battery Be Made From Alkaline Cells A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. Batteries provide electrical energy from chemical energy. The exception is the 9v battery which actually has six smaller cells inside of it. Potassium or sodium hydroxide is the alkaline.. How Can A 6V Battery Be Made From Alkaline Cells.
From www.vynova-group.com
Powering Alkaline Batteries with Potassium Hydroxide How Can A 6V Battery Be Made From Alkaline Cells Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. Batteries provide electrical energy from chemical energy. The exception is the 9v battery which actually has six smaller cells inside of it. The process starts with a one piece metal case which. Potassium or sodium hydroxide is the alkaline. A fully discharged alkaline cell (nominal voltage 1.5. How Can A 6V Battery Be Made From Alkaline Cells.
From www.slideserve.com
PPT Galvanic and Electrolytic Cells PowerPoint Presentation, free How Can A 6V Battery Be Made From Alkaline Cells Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. The exception is the 9v battery which actually has six smaller cells inside of it. A number of cells can be connected in series to make a. Potassium or sodium hydroxide is the alkaline. The process starts with a one piece metal case. How Can A 6V Battery Be Made From Alkaline Cells.
From www.youtube.com
How Does a Battery Work? Alkaline Batteries AP Chemistry YouTube How Can A 6V Battery Be Made From Alkaline Cells In this video we’ll look at how alkaline based batteries are made. The process starts with a one piece metal case which. Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to. How Can A 6V Battery Be Made From Alkaline Cells.
From www.electricity-magnetism.org
Characteristics of Alkaline Batteries Cell voltage, Capacity & Self How Can A 6V Battery Be Made From Alkaline Cells , which has a higher voltage than a. In this video we’ll look at how alkaline based batteries are made. A simple cell can be made by connecting two different metals in contact with an electrolyte. Potassium or sodium hydroxide is the alkaline. The exception is the 9v battery which actually has six smaller cells inside of it. A number. How Can A 6V Battery Be Made From Alkaline Cells.
From electrical4u.com
Alkaline Batteries Electrical4u How Can A 6V Battery Be Made From Alkaline Cells The process starts with a one piece metal case which. Batteries provide electrical energy from chemical energy. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. Potassium or sodium hydroxide is the alkaline. A simple cell can be made by connecting two different metals in contact with an electrolyte. All of the common disposable alkaline batteries. How Can A 6V Battery Be Made From Alkaline Cells.
From batteryguy.com
What are Alkaline Batteries? Knowledge Base How Can A 6V Battery Be Made From Alkaline Cells All of the common disposable alkaline batteries from aaa to d are single cells. Batteries provide electrical energy from chemical energy. A simple cell can be made by connecting two different metals in contact with an electrolyte. Potassium or sodium hydroxide is the alkaline. The process starts with a one piece metal case which. Thus, the chemical composition inside the. How Can A 6V Battery Be Made From Alkaline Cells.
From www.aliexpress.com
10pcs 4AG13 28A 544 L1325 4A76 6V Dry Alkaline Batteries Cells for How Can A 6V Battery Be Made From Alkaline Cells Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. In this video we’ll look at how alkaline based batteries are made. Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. All of the common disposable alkaline batteries from aaa. How Can A 6V Battery Be Made From Alkaline Cells.
From www.homedepot.com
Energizer Alkaline 6Volt Battery529 The Home Depot How Can A 6V Battery Be Made From Alkaline Cells Potassium or sodium hydroxide is the alkaline. Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. The process starts with a one piece metal case which. In this video we’ll look at how alkaline based batteries are made. , which has a higher voltage than a. A simple cell can be made. How Can A 6V Battery Be Made From Alkaline Cells.
From wbminternational.com
Alkaline Battery Cells 2 Pcs How Can A 6V Battery Be Made From Alkaline Cells Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. The process starts with a one piece metal case which. All of the common disposable alkaline batteries from aaa to d are single cells. A number of cells can be connected in series to make a. A simple cell can be made by connecting two different metals. How Can A 6V Battery Be Made From Alkaline Cells.
From www.batterymart.com
6 Volt 180 Ah Gel Cell Sealed Lead Acid Battery Battery Mart How Can A 6V Battery Be Made From Alkaline Cells The exception is the 9v battery which actually has six smaller cells inside of it. All of the common disposable alkaline batteries from aaa to d are single cells. The process starts with a one piece metal case which. Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. A fully discharged alkaline cell (nominal voltage 1.5. How Can A 6V Battery Be Made From Alkaline Cells.
From www.electricity-magnetism.org
Chemistry of Alkaline Batteries Chemical reaction Electricity How Can A 6V Battery Be Made From Alkaline Cells , which has a higher voltage than a. All of the common disposable alkaline batteries from aaa to d are single cells. Batteries provide electrical energy from chemical energy. Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. A fully discharged alkaline cell (nominal voltage 1.5 volts). How Can A 6V Battery Be Made From Alkaline Cells.
From www.vlad.fr
Battery alkaline 4LR25 Duracell 6V Vlad How Can A 6V Battery Be Made From Alkaline Cells A number of cells can be connected in series to make a. Batteries provide electrical energy from chemical energy. The exception is the 9v battery which actually has six smaller cells inside of it. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts. Thus, the chemical composition inside the battery is. How Can A 6V Battery Be Made From Alkaline Cells.
From www.gosupps.com
Duracell 28A 6V Alkaline Battery, 1 Count Pack, 28A 6 Volt Alkaline How Can A 6V Battery Be Made From Alkaline Cells Alkaline batteries are disposable batteries with electrodes made of zinc and manganese dioxide. A number of cells can be connected in series to make a. A simple cell can be made by connecting two different metals in contact with an electrolyte. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts. The. How Can A 6V Battery Be Made From Alkaline Cells.
From courses.lumenlearning.com
Batteries and Fuel Cells General Chemistry How Can A 6V Battery Be Made From Alkaline Cells All of the common disposable alkaline batteries from aaa to d are single cells. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts. Potassium or sodium hydroxide is the alkaline. Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. A simple cell can. How Can A 6V Battery Be Made From Alkaline Cells.
From pressbooks.bccampus.ca
5.6 Batteries and Fuel Cells Chemistry for Chemical Engineers How Can A 6V Battery Be Made From Alkaline Cells , which has a higher voltage than a. A fully discharged alkaline cell (nominal voltage 1.5 volts) still retains a voltage of 0.9 to 1.0 volts. In this video we’ll look at how alkaline based batteries are made. The process starts with a one piece metal case which. Potassium or sodium hydroxide is the alkaline. Explore the essential elements that. How Can A 6V Battery Be Made From Alkaline Cells.
From www.walmart.com
Energizer Max 529 Alkaline Spring Terminal Lantern Battery, 6V How Can A 6V Battery Be Made From Alkaline Cells In this video we’ll look at how alkaline based batteries are made. The process starts with a one piece metal case which. Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital role in generating. Potassium or sodium hydroxide is the alkaline. Thus, the chemical composition inside the battery is very. How Can A 6V Battery Be Made From Alkaline Cells.
From www.electricity-magnetism.org
6V Battery Type, Size & Characteristics How Can A 6V Battery Be Made From Alkaline Cells A number of cells can be connected in series to make a. In this video we’ll look at how alkaline based batteries are made. Thus, the chemical composition inside the battery is very crucial for the perfect functioning of a battery. Explore the essential elements that make up a seemingly simple yet intricate 6 volt battery, each playing a vital. How Can A 6V Battery Be Made From Alkaline Cells.