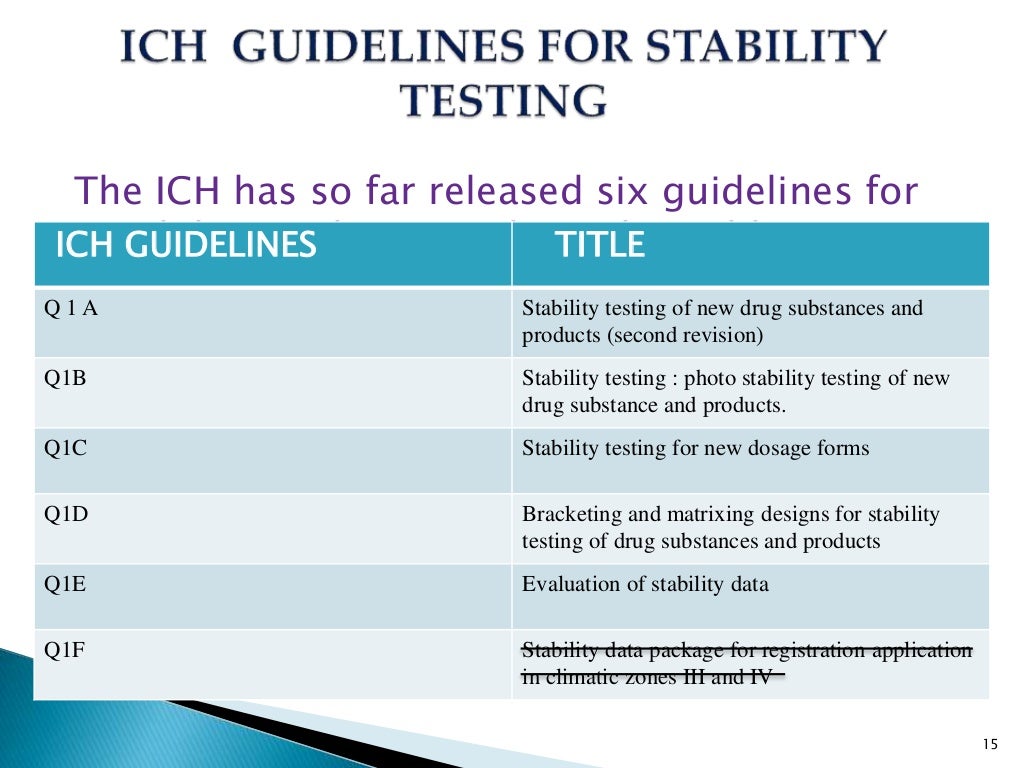
Ich Guidelines Shelf Life Extension . This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. Retest period or shelf life. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february.
from www.vrogue.co
Retest period or shelf life. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest.
Stability Study Sop As Per Ich Guideline Pharma Begin vrogue.co
Ich Guidelines Shelf Life Extension The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. Retest period or shelf life. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a.
From www.researchgate.net
(PDF) Application of Edible Coating in Extension of Fruit Shelf Life Ich Guidelines Shelf Life Extension Retest period or shelf life. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can. Ich Guidelines Shelf Life Extension.
From www.researchgate.net
(PDF) Shelf life extension of bread using ethanol emitters with Ich Guidelines Shelf Life Extension The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a.. Ich Guidelines Shelf Life Extension.
From www.scribd.com
ICH Q1 Stability Guidelines PDF Shelf Life Ich Guidelines Shelf Life Extension The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a.. Ich Guidelines Shelf Life Extension.
From www.researchgate.net
(PDF) Shelf Life Extension of Chicken Cuts Packed under Modified Ich Guidelines Shelf Life Extension Retest period or shelf life. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed. Ich Guidelines Shelf Life Extension.
From www.pharmoutsourcing.com
Accelerated Stability Assessment Program (ASAP) Using Science to Set Ich Guidelines Shelf Life Extension This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest.. Ich Guidelines Shelf Life Extension.
From issuu.com
COVID19 CareStart Antigen Test ShelfLife Extension Notification by Ich Guidelines Shelf Life Extension Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. Retest period or shelf life. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed. Ich Guidelines Shelf Life Extension.
From foodsafetystandard.in
Food Safety Standard Ich Guidelines Shelf Life Extension This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. Retest period or shelf life. Having reached step 4 of the ich process at the ich steering. Ich Guidelines Shelf Life Extension.
From pharmabeej.com
ICH Stability Guidelines Q1A Q1F Pharmabeej Ich Guidelines Shelf Life Extension This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This. Ich Guidelines Shelf Life Extension.
From www.mdpi.com
Shelf Life Extension and Nutritional Quality Preservation of Sour Ich Guidelines Shelf Life Extension This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. Retest period or shelf life. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. Having reached step 4 of the ich process at the ich steering. Ich Guidelines Shelf Life Extension.
From www.dobbsferry.com
IHEALTH COVID19 TEST KIT SHELF LIFE EXTENSION INFORMATION Village of Ich Guidelines Shelf Life Extension Retest period or shelf life. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2). Ich Guidelines Shelf Life Extension.
From www.youtube.com
Shelf Life Extension For Sterile Medical Devices STERIS AST TechTalk Ich Guidelines Shelf Life Extension Retest period or shelf life. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. The purpose of the ich q1e guideline is to provide recommendations on. Ich Guidelines Shelf Life Extension.
From www.researchgate.net
Shelf life extension by biopolymer‐based packaging [26, 227, 228 Ich Guidelines Shelf Life Extension The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2). Ich Guidelines Shelf Life Extension.
From www.aplyon.com
Shelf Life Procedure Ich Guidelines Shelf Life Extension Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. Retest period or shelf life. This document explains how to use stability data generated in accordance with the ich guideline q1a. Ich Guidelines Shelf Life Extension.
From www.slideserve.com
PPT shelflife extension pest disinfestation microbial Ich Guidelines Shelf Life Extension The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. Retest period or shelf life. This document explains how to use stability data generated in accordance with. Ich Guidelines Shelf Life Extension.
From www.scribd.com
Ich Stability Guidelines Final PDF Shelf Life Ultraviolet Ich Guidelines Shelf Life Extension Retest period or shelf life. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can. Ich Guidelines Shelf Life Extension.
From www.foodresearchlab.com
Regulations in Shelf Life Extension of Food Products Ich Guidelines Shelf Life Extension This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february.. Ich Guidelines Shelf Life Extension.
From q1scientific.com
A Q&A guide to stability storage Q1 Scientific Ich Guidelines Shelf Life Extension This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. Retest period or shelf life. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. The purpose of the ich q1e guideline is to provide recommendations on. Ich Guidelines Shelf Life Extension.
From www.slideshare.net
Guide to DuPont™ Danisco® shelf life extension solutions Ich Guidelines Shelf Life Extension Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. Retest period or shelf life. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed. Ich Guidelines Shelf Life Extension.
From www.galdi.it
How to Extend the Shelf Life of Food Products Galdi Ich Guidelines Shelf Life Extension This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. Retest period or shelf life. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be. Ich Guidelines Shelf Life Extension.
From www.vrogue.co
Stability Study Sop As Per Ich Guideline Pharma Begin vrogue.co Ich Guidelines Shelf Life Extension The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. Retest period or shelf life. Having reached step 4 of the ich process at the ich steering. Ich Guidelines Shelf Life Extension.
From www.slideserve.com
PPT Shelf Life Extension Program (SLEP) PowerPoint Presentation, free Ich Guidelines Shelf Life Extension The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. This. Ich Guidelines Shelf Life Extension.
From www.galdi.it
How to Extend the Shelf Life of Food Products Galdi Ich Guidelines Shelf Life Extension Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a.. Ich Guidelines Shelf Life Extension.
From pharmasciences.in
ICH Guideline Q1A (R2) Ich Guidelines Shelf Life Extension Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. Retest period or shelf life. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. This document explains how to use stability data generated in accordance with the ich guideline q1a. Ich Guidelines Shelf Life Extension.
From www.frontiersin.org
Frontiers Recent insights into metallic nanoparticles in shelflife Ich Guidelines Shelf Life Extension This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. Retest period or shelf life. This document explains how to use stability data generated in accordance with. Ich Guidelines Shelf Life Extension.
From www.researchgate.net
(PDF) Comparison of shelflife extension of capsicum (Capsicum annum Ich Guidelines Shelf Life Extension This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2). Ich Guidelines Shelf Life Extension.
From www.youtube.com
Stability Study II ICH Guideline Q1A(R2) II ICH Guideline Q1E II Shelf Ich Guidelines Shelf Life Extension Retest period or shelf life. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This guidance provides recommendations on how to use stability data generated in accordance. Ich Guidelines Shelf Life Extension.
From www.researchgate.net
3 Methods available for shelflife extension of minimally processed Ich Guidelines Shelf Life Extension The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. Retest period or shelf life. This document explains how to use stability data generated in accordance with. Ich Guidelines Shelf Life Extension.
From www.semanticscholar.org
[PDF] ICH guidelines inception, revision, and implications for drug Ich Guidelines Shelf Life Extension This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. Retest. Ich Guidelines Shelf Life Extension.
From www.scommerce-mage.com
Shelf Life extension for Magento 2 ERP Define Product Expiry Date Ich Guidelines Shelf Life Extension This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. Ich Guidelines Shelf Life Extension.
From www.scribd.com
Guideline Quality Aspects Included Product Information Vaccines Human Ich Guidelines Shelf Life Extension Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a.. Ich Guidelines Shelf Life Extension.
From www.amazon.com
Preservation and Shelf Life Extension UV Applications for Fluid Foods Ich Guidelines Shelf Life Extension Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. Retest period or shelf life. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a. This document explains how to use stability data generated in accordance with the ich guideline q1a. Ich Guidelines Shelf Life Extension.
From pharmabeej.com
Q1A (R2) Stability ICH Guideline Purpose Of Revision Pharmabeej Ich Guidelines Shelf Life Extension Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in the ich guidance q1a.. Ich Guidelines Shelf Life Extension.
From www.researchgate.net
(PDF) Shelf Life Extension of Ethylene Treated Bananas at Different Ich Guidelines Shelf Life Extension The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. This guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. Ich Guidelines Shelf Life Extension.
From www.linkedin.com
ICH Q1A Guideline The key to shelf life assessment of pharmaceutical Ich Guidelines Shelf Life Extension Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be used to establish retest. Retest. Ich Guidelines Shelf Life Extension.
From www.dobbsferry.com
IHEALTH COVID19 TEST KIT SHELF LIFE EXTENSION INFORMATION Village of Ich Guidelines Shelf Life Extension Retest period or shelf life. This document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. Having reached step 4 of the ich process at the ich steering committee meeting on 6 february. The purpose of the ich q1e guideline is to provide recommendations on how stability data can be. Ich Guidelines Shelf Life Extension.