What Is Total Hardness . Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Water hardness units express, in most basic terms, the sum. Any reading over 150+ ppm indicates hard water. The most common polyvalent cations in fresh water are calcium (ca ++) and. Total hardness is a measurement of the mineral content in a water sample that is irreversible by. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). What does total hardness mean? The higher the number, the harder your. Hard water can cause mineral buildup in. Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Hardness is a property of water that is not a health concern, but it can be a nuisance.
from mavink.com
Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Hardness is a property of water that is not a health concern, but it can be a nuisance. Hard water can cause mineral buildup in. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). The higher the number, the harder your. What does total hardness mean? Any reading over 150+ ppm indicates hard water. The most common polyvalent cations in fresh water are calcium (ca ++) and. Water hardness units express, in most basic terms, the sum.
Water Hardness Conversion Chart
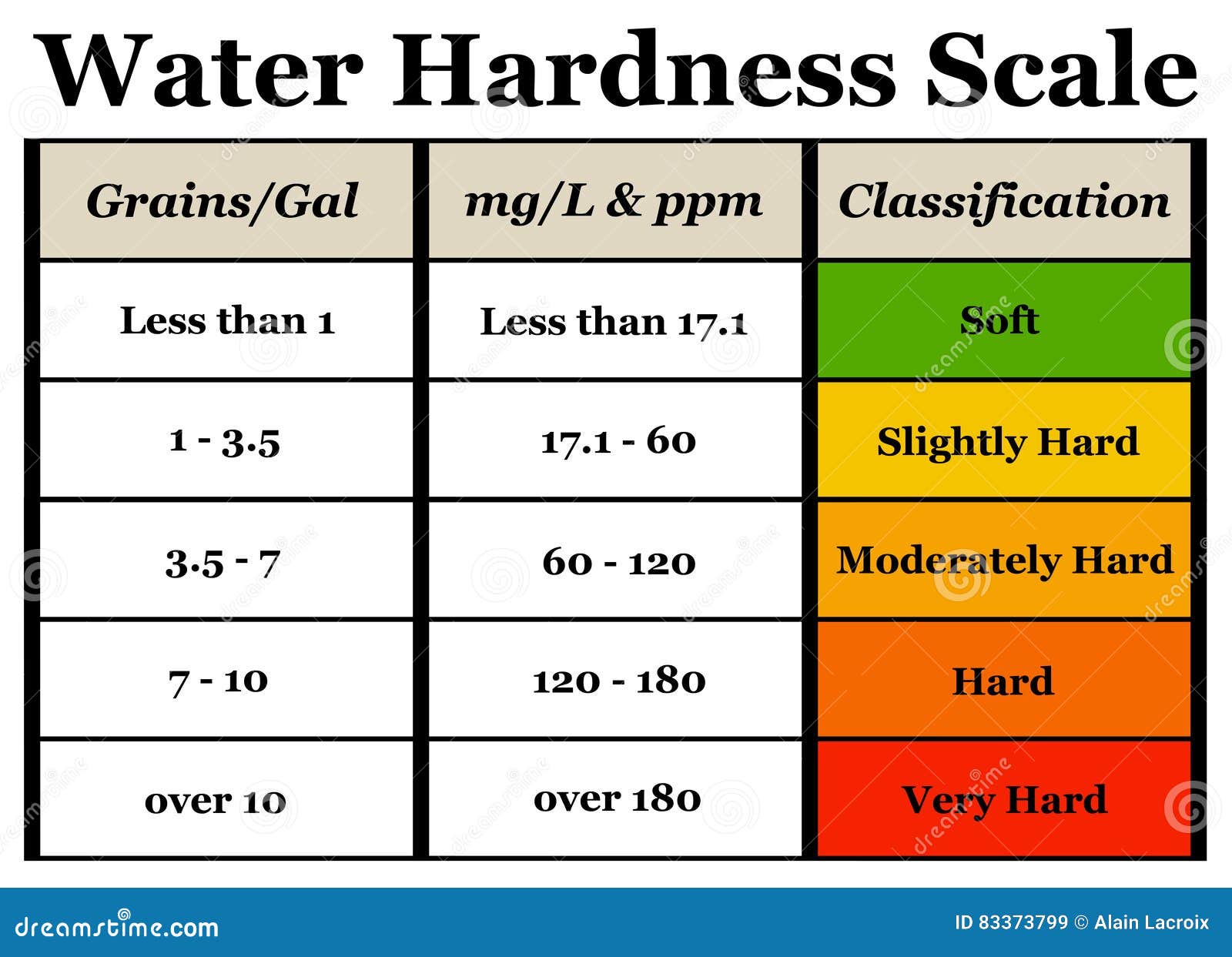
What Is Total Hardness Hardness is a property of water that is not a health concern, but it can be a nuisance. The higher the number, the harder your. The most common polyvalent cations in fresh water are calcium (ca ++) and. Total hardness is a measurement of the mineral content in a water sample that is irreversible by. Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). What does total hardness mean? Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Water hardness units express, in most basic terms, the sum. Hard water can cause mineral buildup in. Any reading over 150+ ppm indicates hard water. Hardness is a property of water that is not a health concern, but it can be a nuisance.
From www.hannainst.com
Total Hardness Colorimetric Reagents (100 tests) HI937350 What Is Total Hardness Hard water can cause mineral buildup in. Any reading over 150+ ppm indicates hard water. What does total hardness mean? The most common polyvalent cations in fresh water are calcium (ca ++) and. Hardness is a property of water that is not a health concern, but it can be a nuisance. Water hardness units express, in most basic terms, the. What Is Total Hardness.
From www.globalseafood.org
Total alkalinity and total hardness Responsible Seafood Advocate What Is Total Hardness What does total hardness mean? Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Hard water can cause mineral buildup in. Hardness is a property of water that is not a health concern, but it can be a nuisance. The higher the number, the harder your. Total hardness is defined as the total. What Is Total Hardness.
From sereneaquarium.com.au
Water Hardness Serene Aquarium What Is Total Hardness The higher the number, the harder your. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the. What Is Total Hardness.
From www.chegg.com
Solved a) What is total hardness? b) What is carbonate What Is Total Hardness The higher the number, the harder your. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Hardness is a property of water that is not a health concern, but it can be a nuisance. What does total hardness mean? Total hardness is a measurement of the mineral content in. What Is Total Hardness.
From www.slideserve.com
PPT Hardness PowerPoint Presentation, free download ID815705 What Is Total Hardness Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. The higher the number, the harder your. Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium. What Is Total Hardness.
From www.researchgate.net
The graph of TDS vs TH (total hardness) in water Download Scientific What Is Total Hardness Any reading over 150+ ppm indicates hard water. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). What does total hardness mean? Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Hard water can cause mineral buildup in. Water hardness units express, in. What Is Total Hardness.
From studylib.net
Carbonate hardness What Is Total Hardness Any reading over 150+ ppm indicates hard water. What does total hardness mean? Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). Hardness is a property of water that is not a health concern, but it can be a nuisance. Total hardness is defined as the total concentration of divalent. What Is Total Hardness.
From www.wahlwater.com
Total Dissolved Solids Jeff Wahl What Is Total Hardness The most common polyvalent cations in fresh water are calcium (ca ++) and. Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). What does total hardness mean? Chemically, hardness is often defined. What Is Total Hardness.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube What Is Total Hardness What does total hardness mean? Hardness is a property of water that is not a health concern, but it can be a nuisance. The most common polyvalent cations in fresh water are calcium (ca ++) and. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Water hardness units express,. What Is Total Hardness.
From www.beanscenemag.com.au
Water hardness made easy BeanScene What Is Total Hardness The higher the number, the harder your. Hard water can cause mineral buildup in. The most common polyvalent cations in fresh water are calcium (ca ++) and. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). Water hardness units express, in most basic terms, the sum. Namely, total water hardness. What Is Total Hardness.
From itechnhealth.com
How Do You Know If You Have Hard Water What Is Total Hardness Water hardness units express, in most basic terms, the sum. The higher the number, the harder your. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Namely, total water hardness is caused. What Is Total Hardness.
From waternitylab.com
Water Hardness Scale GPG, mmol/L, PPM Chart What Is Total Hardness Any reading over 150+ ppm indicates hard water. What does total hardness mean? Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). The higher the number, the harder your. Hard water can cause mineral buildup in. Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in. What Is Total Hardness.
From www.nsilabsolutions.com
Total Hardness CRM, 1000mg/L, PartIS017 NSI Lab Solutions What Is Total Hardness Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. The most common polyvalent cations in fresh water are calcium (ca ++) and. Any reading over 150+ ppm indicates hard water. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). Hard water. What Is Total Hardness.
From www.numerade.com
SOLVED Calculate total hardness, carbonate hardness, and noncarbonate What Is Total Hardness The most common polyvalent cations in fresh water are calcium (ca ++) and. Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. The higher the number, the harder your. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). Namely, total water. What Is Total Hardness.
From mavink.com
Water Hardness Conversion Chart What Is Total Hardness The higher the number, the harder your. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Any reading over 150+ ppm indicates hard water. Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Water hardness units express, in most basic. What Is Total Hardness.
From www.slideserve.com
PPT Alkalinity and hardness PowerPoint Presentation, free download What Is Total Hardness Hardness is a property of water that is not a health concern, but it can be a nuisance. The higher the number, the harder your. Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Hard. What Is Total Hardness.
From www.safewater.org
Total Hardness Analysis Instructions for Elementary in PowerPoint and What Is Total Hardness Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). Hard water can cause mineral buildup in. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions. What Is Total Hardness.
From www.ysi.com
Water Hardness Measurements What Is Total Hardness Total hardness is a measurement of the mineral content in a water sample that is irreversible by. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Water hardness units express, in most basic terms, the sum. Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved. What Is Total Hardness.
From www.researchgate.net
PH, total hardness, EC, TDS, major ions and temperature for water What Is Total Hardness The higher the number, the harder your. Hard water can cause mineral buildup in. What does total hardness mean? Total hardness is a measurement of the mineral content in a water sample that is irreversible by. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Water hardness is measured. What Is Total Hardness.
From www.westernvawater.org
Water Hardness Western Virginia Water Authority What Is Total Hardness Hard water can cause mineral buildup in. Any reading over 150+ ppm indicates hard water. Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Hardness is a property of water that is not a health concern, but it can be a nuisance. Chemically, hardness is often defined as the sum of. What Is Total Hardness.
From www.watertestsystems.com.au
TOTAL HARDNESS TABS BOTTLE What Is Total Hardness Water hardness units express, in most basic terms, the sum. The higher the number, the harder your. Hard water can cause mineral buildup in. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Total. What Is Total Hardness.
From www.slideserve.com
PPT MECHANICAL PROPERTIES PowerPoint Presentation, free download ID What Is Total Hardness Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Water hardness units express, in most basic terms, the sum. Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. What does total hardness mean? The most common polyvalent cations in fresh. What Is Total Hardness.
From www.youtube.com
Calculation of Total Hardness Total Hardness Total Hardness of What Is Total Hardness Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. The most common polyvalent cations in. What Is Total Hardness.
From sciencenotes.org
Mohs Hardness Scale What Is Total Hardness Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Water hardness units express, in most basic terms, the sum. Total hardness is a measurement of the mineral content in a water sample that is irreversible by. Hardness is a property of water that is not a health concern, but it can. What Is Total Hardness.
From waterfilterspot.com
Water Hardness Scale For Water Softener The Ideal Number What Is Total Hardness Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). Hard water can cause mineral buildup in. Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. The most common polyvalent cations in fresh water are calcium (ca ++) and. Total hardness is. What Is Total Hardness.
From www.slideserve.com
PPT Water hardness & Special treatment PowerPoint Presentation ID What Is Total Hardness Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Water hardness units express, in most basic terms, the sum. Hard water can cause mineral buildup in. Total hardness is a measurement of the mineral content. What Is Total Hardness.
From mavink.com
Water Hardness Conversion Chart What Is Total Hardness What does total hardness mean? Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). The most common polyvalent cations in fresh water are calcium (ca ++) and. Water hardness is measured in terms. What Is Total Hardness.
From www.slideserve.com
PPT Hardness of Water PowerPoint Presentation, free download ID2279522 What Is Total Hardness Total hardness is a measurement of the mineral content in a water sample that is irreversible by. Total hardness is defined as the total concentration of divalent cations in water expressed as equivalent calcium carbonate. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). What does total hardness mean? The. What Is Total Hardness.
From www.slideserve.com
PPT Hardness PowerPoint Presentation, free download ID2261322 What Is Total Hardness The most common polyvalent cations in fresh water are calcium (ca ++) and. The higher the number, the harder your. Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). Hardness is a property of water that is not a health concern, but it can be a nuisance. Namely, total water. What Is Total Hardness.
From waterfilterguru.com
What is the Ideal Water Hardness Level? Water Filter Guru What Is Total Hardness The most common polyvalent cations in fresh water are calcium (ca ++) and. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Any reading over 150+ ppm indicates hard water. Total hardness is a measurement of the mineral content in a water sample that is irreversible by. The higher. What Is Total Hardness.
From www.slideshare.net
Determination of hardness of water What Is Total Hardness Hard water can cause mineral buildup in. What does total hardness mean? Any reading over 150+ ppm indicates hard water. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). The higher the number, the harder your. Total hardness is a measurement of the mineral content in a water sample. What Is Total Hardness.
From www.ibuychemikals.com
Buy Total Hardness Indicator Tablets online in India at best price What Is Total Hardness Total hardness is a measurement of the mineral content in a water sample that is irreversible by. Hardness is a property of water that is not a health concern, but it can be a nuisance. Hard water can cause mineral buildup in. The most common polyvalent cations in fresh water are calcium (ca ++) and. Namely, total water hardness is. What Is Total Hardness.
From water.mecc.edu
Hardness What Is Total Hardness Water hardness units express, in most basic terms, the sum. What does total hardness mean? Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/l). The higher the number, the harder your. Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg. What Is Total Hardness.
From www.researchgate.net
Total alkalinity, total hardness, chloride content, BOD5, COD What Is Total Hardness Total hardness is a measurement of the mineral content in a water sample that is irreversible by. Hard water can cause mineral buildup in. Water hardness units express, in most basic terms, the sum. Hardness is a property of water that is not a health concern, but it can be a nuisance. Total hardness is defined as the total concentration. What Is Total Hardness.
From www.forestry-suppliers.com
Hach® Total Hardness Test Strips What Is Total Hardness What does total hardness mean? Namely, total water hardness is caused by the presence of both calcium ions (ca 2+) and magnesium ions (mg 2). Chemically, hardness is often defined as the sum of polyvalent cation concentrations dissolved in the water. Hardness is a property of water that is not a health concern, but it can be a nuisance. Total. What Is Total Hardness.