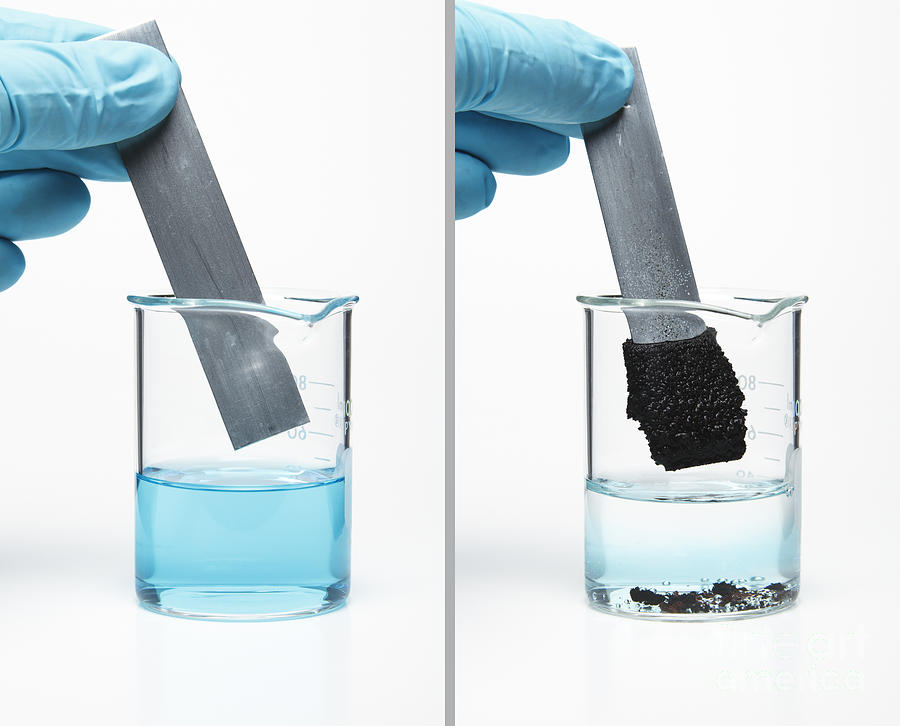
A Zinc Rod Was Dipped In Cuso4 . Nitrous acid h n o 2. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper. 0.5 or 1m copper sulfate; These types of reactions are called direct redox reactions because the. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. A piece of zinc is placed in a copper sulfate solution. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution.
from fineartamerica.com
As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper. Nitrous acid h n o 2. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. A piece of zinc is placed in a copper sulfate solution. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. 0.5 or 1m copper sulfate; These types of reactions are called direct redox reactions because the.
Zinc Reacting With Copper Sulfate Photograph by GIPhotoStock
A Zinc Rod Was Dipped In Cuso4 As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. These types of reactions are called direct redox reactions because the. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. 0.5 or 1m copper sulfate; Nitrous acid h n o 2. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. A piece of zinc is placed in a copper sulfate solution. When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the.
From www.youtube.com
A zinc rod is dipped in \( 0.1 \mathrm{M} \) solution of \( \mathrm... YouTube A Zinc Rod Was Dipped In Cuso4 0.5 or 1m copper sulfate; Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. As zinc is more reactive than copper, zinc will displace copper from its solution. A Zinc Rod Was Dipped In Cuso4.
From www.youtube.com
Zinc (Zn) reaction with Copper Sulphate (CuSO4) Displacement Reaction Chemistry YouTube A Zinc Rod Was Dipped In Cuso4 0.5 or 1m copper sulfate; A piece of zinc is placed in a copper sulfate solution. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. When a. A Zinc Rod Was Dipped In Cuso4.
From fineartamerica.com
Zinc Reacting With Copper Sulfate Photograph by GIPhotoStock A Zinc Rod Was Dipped In Cuso4 As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. A piece of zinc is placed in a copper sulfate solution. Zinc (zn) is more. A Zinc Rod Was Dipped In Cuso4.
From www.slideshare.net
Replacement reactions A Zinc Rod Was Dipped In Cuso4 As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. A piece of zinc is placed in a copper sulfate solution. When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper. It is commonly. A Zinc Rod Was Dipped In Cuso4.
From www.youtube.com
A cell is prepared by dipping copper rod in 1 M copper sulphate solution and zinc rod in 1 M zin A Zinc Rod Was Dipped In Cuso4 Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. A piece of zinc is placed in a copper sulfate solution. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. When a zinc rod is dipped in a. A Zinc Rod Was Dipped In Cuso4.
From www.youtube.com
Zinc and Copper Sulphate reaction (Displacement Reaction) Zn and CuSO4 YouTube A Zinc Rod Was Dipped In Cuso4 0.5 or 1m copper sulfate; Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. A piece of zinc is placed in a copper sulfate solution.. A Zinc Rod Was Dipped In Cuso4.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians A Zinc Rod Was Dipped In Cuso4 Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Nitrous acid h n o 2. It is commonly known that when zinc metal is placed in a. A Zinc Rod Was Dipped In Cuso4.
From www.meritnation.com
wt happened when a zinc strip is dipped in CuSO4 solution a) write the eqn for the Science A Zinc Rod Was Dipped In Cuso4 Nitrous acid h n o 2. These types of reactions are called direct redox reactions because the. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. A piece of zinc is placed in a copper sulfate solution. It is commonly known that when zinc metal is placed in. A Zinc Rod Was Dipped In Cuso4.
From www.meritnation.com
wt happened when a zinc strip is dipped in CuSO4 solution a) write the eqn for the Science A Zinc Rod Was Dipped In Cuso4 When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper. These types of reactions are called direct redox reactions because the. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons.. A Zinc Rod Was Dipped In Cuso4.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID4435141 A Zinc Rod Was Dipped In Cuso4 A piece of zinc is placed in a copper sulfate solution. When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper. These types of reactions are called direct redox reactions because the. It is commonly known that when zinc metal is placed in a solution. A Zinc Rod Was Dipped In Cuso4.
From www.doubtnut.com
Raju found that when a zinc rod is immersed in CuSo4 solution taken in A Zinc Rod Was Dipped In Cuso4 It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. These types of reactions are called direct redox reactions because the. When. A Zinc Rod Was Dipped In Cuso4.
From askfilo.com
When a zinc rod is placed in an aqueous solution of CuSO4 , the following.. A Zinc Rod Was Dipped In Cuso4 These types of reactions are called direct redox reactions because the. 0.5 or 1m copper sulfate; A piece of zinc is placed in a copper sulfate solution. When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper. Ions of any metal that is below zinc,. A Zinc Rod Was Dipped In Cuso4.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps A Zinc Rod Was Dipped In Cuso4 These types of reactions are called direct redox reactions because the. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. It is commonly known that when zinc metal. A Zinc Rod Was Dipped In Cuso4.
From www.shalom-education.com
Displacement Reactions GCSE Chemistry Revision A Zinc Rod Was Dipped In Cuso4 These types of reactions are called direct redox reactions because the. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. A piece of zinc is placed in a copper sulfate solution. Nitrous acid h n o 2. It is commonly known that when zinc metal is placed in. A Zinc Rod Was Dipped In Cuso4.
From blog.thepipingmart.com
Zinc Displacing Copper in CuSO4 Solutions A Zinc Rod Was Dipped In Cuso4 Nitrous acid h n o 2. These types of reactions are called direct redox reactions because the. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. 0.5 or 1m copper sulfate; As zinc is more reactive than copper, zinc will displace copper from its solution. A Zinc Rod Was Dipped In Cuso4.
From socratic.org
The color of light absorbed by an aqueous solution of CuSO_"4" is?? Socratic A Zinc Rod Was Dipped In Cuso4 Nitrous acid h n o 2. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. These types of reactions are called direct redox reactions because the. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4). A Zinc Rod Was Dipped In Cuso4.
From www.youtube.com
(a) A cell is prepared by dipping a zinc rod in 1M zinc sulphate solution and a silver electrode A Zinc Rod Was Dipped In Cuso4 Nitrous acid h n o 2. A piece of zinc is placed in a copper sulfate solution. These types of reactions are called direct redox reactions because the. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. When a zinc rod is dipped in a solution of copper. A Zinc Rod Was Dipped In Cuso4.
From brainly.in
Vidisha placed a zinc plate in copper sulphate solution as shown in the figure . Which of the A Zinc Rod Was Dipped In Cuso4 When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. A piece of zinc is placed in a copper sulfate solution. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. These types of reactions are. A Zinc Rod Was Dipped In Cuso4.
From byjus.com
Daniell Cell Definition, Construction & Working with Cell Reactions A Zinc Rod Was Dipped In Cuso4 These types of reactions are called direct redox reactions because the. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. When. A Zinc Rod Was Dipped In Cuso4.
From www.youtube.com
️ Zn in CuSO4 sln YouTube A Zinc Rod Was Dipped In Cuso4 When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. A piece of zinc is placed in a copper sulfate solution. These types of reactions are called direct redox reactions because the. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from. A Zinc Rod Was Dipped In Cuso4.
From www.youtube.com
Q39. (a) A zinc rod weighing 25.00 g was kept in 100 mL of 1 M CuSO4 solution. After a certain A Zinc Rod Was Dipped In Cuso4 Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the. A Zinc Rod Was Dipped In Cuso4.
From www.toppr.com
When copper rod is dipped in CuSO4 solution, the potential developed at the interface is called as A Zinc Rod Was Dipped In Cuso4 As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. Nitrous acid h n o 2. A piece of zinc is placed in a copper sulfate solution. Zinc. A Zinc Rod Was Dipped In Cuso4.
From www.youtube.com
Zn+CuSO4=Cu+ZnSO4 balance the displacement reactionmydocumentary838 YouTube A Zinc Rod Was Dipped In Cuso4 When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. These types of reactions are called direct redox reactions because the. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. When a zinc rod. A Zinc Rod Was Dipped In Cuso4.
From bertigamas.github.io
Zn Cuso4 Znso4 Cu Brain A Zinc Rod Was Dipped In Cuso4 0.5 or 1m copper sulfate; Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. A piece of zinc is placed in a copper sulfate. A Zinc Rod Was Dipped In Cuso4.
From www.vrogue.co
Solved With The Aid Of A Clearly Labelled Diagram Des vrogue.co A Zinc Rod Was Dipped In Cuso4 Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Nitrous acid h n o 2. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. When a zinc rod is dipped in a cuso4 solution, the zinc atoms. A Zinc Rod Was Dipped In Cuso4.
From www.youtube.com
single displacement reaction of Copper from Copper Sulphate Solution by Zinc YouTube A Zinc Rod Was Dipped In Cuso4 As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. 0.5 or 1m copper sulfate; When a zinc rod is dipped in a cuso4 solution, the zinc atoms on. A Zinc Rod Was Dipped In Cuso4.
From www.toppr.com
Zn rod is placed in 100 L of 1M CuSO4 solution so that molarity of Cu^2 + changes to 0.7 M. The A Zinc Rod Was Dipped In Cuso4 These types of reactions are called direct redox reactions because the. When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper. It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is. A Zinc Rod Was Dipped In Cuso4.
From www.youtube.com
What happens when a zinc strip is dipped into a copper sulphate solution ? (a) Write the A Zinc Rod Was Dipped In Cuso4 These types of reactions are called direct redox reactions because the. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. When a zinc rod is dipped in a. A Zinc Rod Was Dipped In Cuso4.
From www.toppr.com
3. A cell is prepared by dipping a copper rod in 1 M CuSO4 solution and a nickel rod in 1M NISO A Zinc Rod Was Dipped In Cuso4 When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. 0.5 or 1m copper sulfate; Ions of any metal that is below zinc, such as. A Zinc Rod Was Dipped In Cuso4.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) A Zinc Rod Was Dipped In Cuso4 When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Nitrous acid h n o 2. 0.5 or 1m copper sulfate; When a zinc rod. A Zinc Rod Was Dipped In Cuso4.
From www.chegg.com
Solved 2) a) When the iron rod was dipped into 5 CuSO4 A Zinc Rod Was Dipped In Cuso4 Nitrous acid h n o 2. A piece of zinc is placed in a copper sulfate solution. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. 0.5 or 1m copper sulfate; As zinc is more reactive than copper, zinc will displace copper from its solution. A Zinc Rod Was Dipped In Cuso4.
From brainly.in
Assertion When zinc rod is dipped into aqueous solution of copper sulphate, the colour of the A Zinc Rod Was Dipped In Cuso4 When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to lose electrons. Nitrous acid h n o 2. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Ions of any metal that is below zinc, such. A Zinc Rod Was Dipped In Cuso4.
From www.youtube.com
Reaction of Copper Sulphate (CuSO4) with Sodium Hydroxide (NaOH) YouTube A Zinc Rod Was Dipped In Cuso4 0.5 or 1m copper sulfate; It is commonly known that when zinc metal is placed in a solution of copper(ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Nitrous acid h n o 2. When a zinc rod is dipped in a cuso4 solution, the zinc atoms on the surface of the rod will start to. A Zinc Rod Was Dipped In Cuso4.
From byjus.com
3. In the electrochemical cell ZnZnSO4(0.01M)CuSO4Cu, the emf of this Daniel cell is E1 A Zinc Rod Was Dipped In Cuso4 Nitrous acid h n o 2. 0.5 or 1m copper sulfate; A piece of zinc is placed in a copper sulfate solution. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc. A Zinc Rod Was Dipped In Cuso4.
From byjus.com
What will happen if Cu rod alone is dipped in cuso4 solution Reduction or oxidation of copper A Zinc Rod Was Dipped In Cuso4 These types of reactions are called direct redox reactions because the. When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper. As zinc is more reactive than copper, zinc will displace copper from its solution and will start forming zinc sulphate solution. Ions of any. A Zinc Rod Was Dipped In Cuso4.