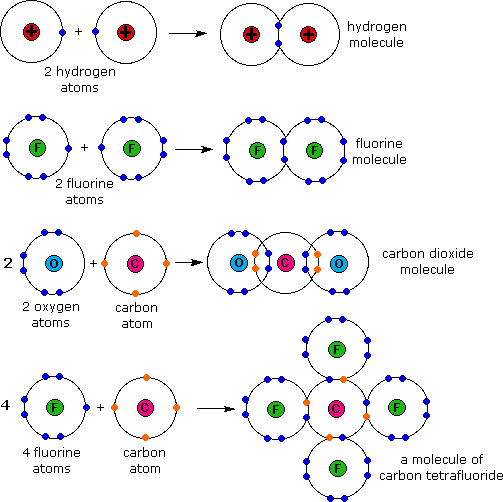
How Do Covalent Bonds Electrons Work . In pure covalent bonds, the. A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. Usually, sharing electrons gives each atom a full valence shell and. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Electronegativity is the ability of an atom to draw electrons to.
from www2.chemistry.msu.edu
Electronegativity is the ability of an atom to draw electrons to. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. Usually, sharing electrons gives each atom a full valence shell and. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. In pure covalent bonds, the. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding.
Electron Configurations & The Periodic Table
How Do Covalent Bonds Electrons Work The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. In pure covalent bonds, the. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. Electronegativity is the ability of an atom to draw electrons to. Usually, sharing electrons gives each atom a full valence shell and. A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID1698471 How Do Covalent Bonds Electrons Work When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. In pure covalent bonds, the. Covalent bonds form when electrons are shared. How Do Covalent Bonds Electrons Work.
From www.science-revision.co.uk
Covalent bonding How Do Covalent Bonds Electrons Work A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. Electronegativity is the ability of an atom to draw electrons to. Usually, sharing electrons gives each atom a full valence shell and. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. How Do Covalent Bonds Electrons Work.
From www.pinterest.co.uk
Covalent Bond Easy Science Covalent bonding, Chemistry basics, Chemistry education How Do Covalent Bonds Electrons Work Electronegativity is the ability of an atom to draw electrons to. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually, sharing electrons gives each atom a full valence shell and. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom. How Do Covalent Bonds Electrons Work.
From mammothmemory.net
Covalent bonding is where atoms share electrons by bonding How Do Covalent Bonds Electrons Work A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher. How Do Covalent Bonds Electrons Work.
From emedia.rmit.edu.au
Covalent bonds Learning Lab How Do Covalent Bonds Electrons Work When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. Electronegativity is the ability of. How Do Covalent Bonds Electrons Work.
From www.science-revision.co.uk
Covalent bonding How Do Covalent Bonds Electrons Work A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond. How Do Covalent Bonds Electrons Work.
From biologydictionary.net
Covalent Bond Biology Dictionary How Do Covalent Bonds Electrons Work Usually, sharing electrons gives each atom a full valence shell and. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. A. How Do Covalent Bonds Electrons Work.
From www.slideserve.com
PPT Covalent bonds where electrons are shared PowerPoint Presentation ID2964935 How Do Covalent Bonds Electrons Work Electronegativity is the ability of an atom to draw electrons to. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. In. How Do Covalent Bonds Electrons Work.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I How Do Covalent Bonds Electrons Work A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. A covalent bond is formed when the electronegativity difference between the two. How Do Covalent Bonds Electrons Work.
From mavink.com
Hydrogen Covalent Bond Diagram How Do Covalent Bonds Electrons Work In pure covalent bonds, the. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for. How Do Covalent Bonds Electrons Work.
From www.youtube.com
Lewis Dot Structures for Covalent Compounds Part 1 CLEAR & SIMPLE YouTube How Do Covalent Bonds Electrons Work A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. Usually, sharing. How Do Covalent Bonds Electrons Work.
From www.pinterest.com
Four covalent bonds. Carbon has four valence electrons and here a valence of four. Each hydrogen How Do Covalent Bonds Electrons Work Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Usually, sharing electrons gives each atom a full valence shell and. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. Electronegativity is the ability of. How Do Covalent Bonds Electrons Work.
From www.teachoo.com
[Class 10] Differentiate between Ionic bond & Covalent bond Teachoo How Do Covalent Bonds Electrons Work In pure covalent bonds, the. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. Usually, sharing electrons gives each atom a full valence shell and. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms.. How Do Covalent Bonds Electrons Work.
From philschatz.com
Chemical Bonds · Anatomy and Physiology How Do Covalent Bonds Electrons Work Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. In pure covalent bonds, the. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the. How Do Covalent Bonds Electrons Work.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts How Do Covalent Bonds Electrons Work A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. Electronegativity is the ability of an atom to draw electrons to. In pure covalent bonds, the. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A covalent bond is a chemical. How Do Covalent Bonds Electrons Work.
From santiagomeowmeyer.blogspot.com
Why Are Only Valence Electrons Involved in Bonding How Do Covalent Bonds Electrons Work The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. In pure covalent bonds, the. Covalent bonds form when electrons are shared between atoms and. How Do Covalent Bonds Electrons Work.
From www.tec-science.com
Covalent bonding tecscience How Do Covalent Bonds Electrons Work Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. Usually, sharing electrons gives each atom a full valence shell and. In pure. How Do Covalent Bonds Electrons Work.
From www.britannica.com
covalent bond Definition, Properties, Examples, & Facts Britannica How Do Covalent Bonds Electrons Work Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually, sharing electrons gives each atom a full valence shell and. Electronegativity is the ability of an atom to draw electrons to. In. How Do Covalent Bonds Electrons Work.
From biologydictionary.net
Covalent Bond Biology Dictionary How Do Covalent Bonds Electrons Work Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. In pure covalent bonds, the. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms. How Do Covalent Bonds Electrons Work.
From kaelyn-well-harmon.blogspot.com
A Covalent Bond Can Best Be Described as How Do Covalent Bonds Electrons Work Electronegativity is the ability of an atom to draw electrons to. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. When atoms of different elements share electrons through covalent bonding, the electron. How Do Covalent Bonds Electrons Work.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.44 Know that a Covalent Bond is Formed Between Atoms by the Sharing of How Do Covalent Bonds Electrons Work A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the. How Do Covalent Bonds Electrons Work.
From www.chemistrystudent.com
Covalent Bonding (ALevel) ChemistryStudent How Do Covalent Bonds Electrons Work Usually, sharing electrons gives each atom a full valence shell and. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. The sharing. How Do Covalent Bonds Electrons Work.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind the Atoms Together How Do Covalent Bonds Electrons Work In pure covalent bonds, the. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding.. How Do Covalent Bonds Electrons Work.
From www.youtube.com
Covalent Bonding DotCross Diagrams GCSE Chemistry Revision YouTube How Do Covalent Bonds Electrons Work A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. Electronegativity is the ability of an atom to draw electrons to. When atoms of different. How Do Covalent Bonds Electrons Work.
From www.youtube.com
Chapter 04 13 Covalent Bonds Sharing the Electrons YouTube How Do Covalent Bonds Electrons Work In pure covalent bonds, the. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A covalent bond is formed when the electronegativity difference between the. How Do Covalent Bonds Electrons Work.
From masterconceptsinchemistry.com
How do atoms form covalent bond? How Do Covalent Bonds Electrons Work The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. A covalent bond is a. How Do Covalent Bonds Electrons Work.
From thescienceandmathszone.com
Covalent Bonding The Science and Maths Zone How Do Covalent Bonds Electrons Work Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. In pure covalent. How Do Covalent Bonds Electrons Work.
From www2.chemistry.msu.edu
Electron Configurations & The Periodic Table How Do Covalent Bonds Electrons Work Usually, sharing electrons gives each atom a full valence shell and. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Electronegativity is the ability of an atom to draw electrons to. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a. How Do Covalent Bonds Electrons Work.
From thesciencecore.blogspot.com
covalent bond definition, properties and examples The Science Core How Do Covalent Bonds Electrons Work A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent. A covalent bond is a chemical bond between two atoms where they. How Do Covalent Bonds Electrons Work.
From users.highland.edu
Lewis Structures and Covalent Bonding How Do Covalent Bonds Electrons Work Usually, sharing electrons gives each atom a full valence shell and. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. In pure covalent bonds, the. The sharing of electrons between atoms. How Do Covalent Bonds Electrons Work.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID5648526 How Do Covalent Bonds Electrons Work A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. A covalent bond is formed when the electronegativity difference between the two atoms is too small (<2) for electron transfer. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually, sharing. How Do Covalent Bonds Electrons Work.
From johelpschemistry.blogspot.ca
ALevel Chemistry 1.6.1c draw electron configuration diagrams of cations and anions using dots How Do Covalent Bonds Electrons Work A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Electronegativity is the ability of an atom to draw electrons to. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. Covalent bonds form when. How Do Covalent Bonds Electrons Work.
From www.chemistrystudent.com
Covalent Bonding (ALevel) ChemistryStudent How Do Covalent Bonds Electrons Work The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. Usually, sharing electrons gives each atom a full valence shell and. Covalent bonds form when. How Do Covalent Bonds Electrons Work.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID2170124 How Do Covalent Bonds Electrons Work Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Usually, sharing electrons gives each atom a full valence shell and. Electronegativity is the ability of an atom to draw electrons to. In pure covalent bonds, the. A polar covalent bond is formed when the nonmetals that share pairs of electrons have. How Do Covalent Bonds Electrons Work.
From worksheetlistix.z13.web.core.windows.net
How To Draw Covalent Bonding How Do Covalent Bonds Electrons Work A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity difference. Usually, sharing electrons gives each atom a full valence shell and. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A covalent bond is formed when the electronegativity difference between the two. How Do Covalent Bonds Electrons Work.