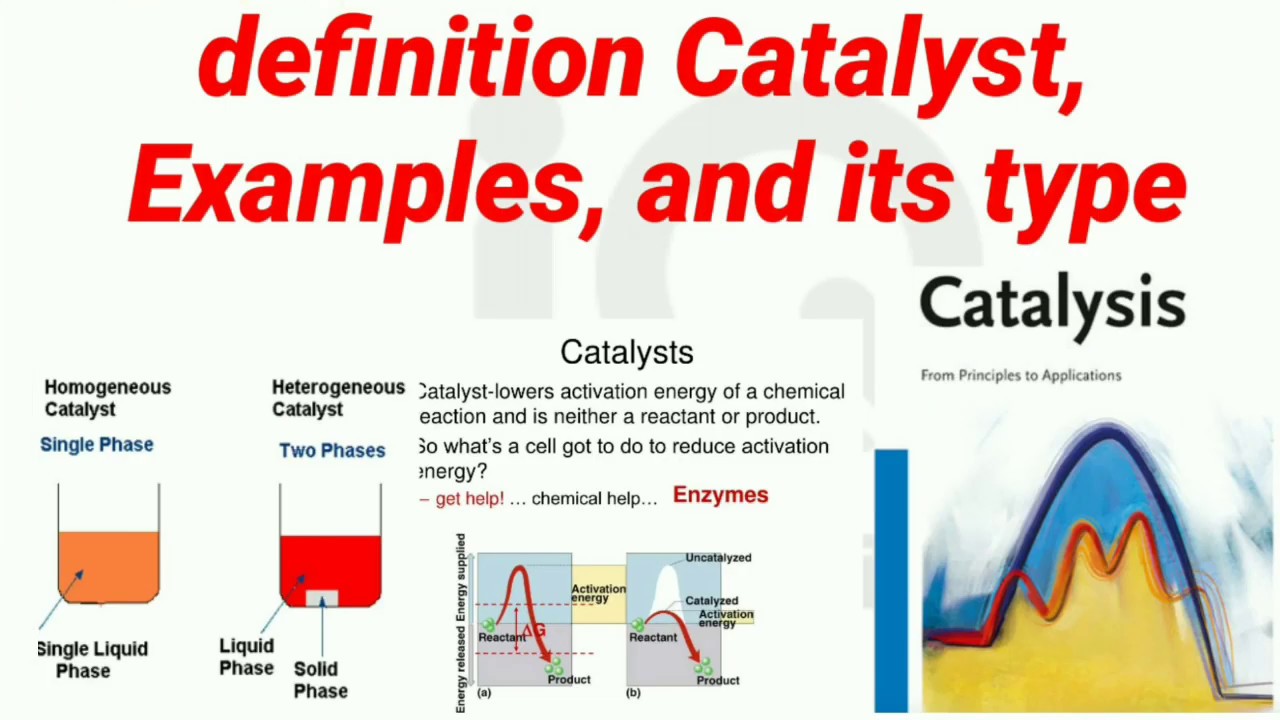
Catalysts Definition Chemistry . catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. In other words, a catalyst is both a. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do. In heterogeneous catalysis, catalysts provide a surface. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Hence a catalyst can be. Each catalyst molecule may induce the transformation of many molecules of reactants.
from medicaltaste.weebly.com
Each catalyst molecule may induce the transformation of many molecules of reactants. Hence a catalyst can be. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; In heterogeneous catalysis, catalysts provide a surface. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is some material that speeds up chemical reactions. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. With a helping hand from a catalyst, molecules that might take years to interact can now do. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy.
Periodic table catalyst definition chemistry medicaltaste
Catalysts Definition Chemistry catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to interact can now do. a catalyst is some material that speeds up chemical reactions. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Each catalyst molecule may induce the transformation of many molecules of reactants. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; In heterogeneous catalysis, catalysts provide a surface. In other words, a catalyst is both a. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. Hence a catalyst can be. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysts Definition Chemistry Each catalyst molecule may induce the transformation of many molecules of reactants. In heterogeneous catalysis, catalysts provide a surface. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; In other words, a catalyst is both a. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by. Catalysts Definition Chemistry.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysts Definition Chemistry catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In other words, a catalyst is both a. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; a catalyst, in turn, is a substance that is. Catalysts Definition Chemistry.
From thechemistrynotes.com
Catalysis Definition, Types, Advantages, Disadvantages Catalysts Definition Chemistry In heterogeneous catalysis, catalysts provide a surface. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. Hence a catalyst can be. a catalyst is some material that speeds up chemical reactions. Each catalyst molecule may induce the transformation of many molecules of reactants. A catalyst. Catalysts Definition Chemistry.
From exorbcrky.blob.core.windows.net
Catalyst Meaning Chem at Rick Franks blog Catalysts Definition Chemistry catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during. Catalysts Definition Chemistry.
From study.com
Catalyst Definition, Types & Function Lesson Catalysts Definition Chemistry catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Each catalyst molecule may induce the transformation of many molecules of reactants. Hence a catalyst can be. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; . Catalysts Definition Chemistry.
From dxxaaiodeco.blob.core.windows.net
Catalyst Chemical Formula at Paul Lowther blog Catalysts Definition Chemistry In heterogeneous catalysis, catalysts provide a surface. Hence a catalyst can be. In other words, a catalyst is both a. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. Catalysts Definition Chemistry.
From cezqijmt.blob.core.windows.net
Catalyst Meaning In Chemistry With Example at Dianna Jackson blog Catalysts Definition Chemistry a catalyst is some material that speeds up chemical reactions. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In other words, a catalyst is both a. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand. Catalysts Definition Chemistry.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalysts Definition Chemistry Hence a catalyst can be. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. With a helping hand from a catalyst, molecules that might take years to interact can now do. In heterogeneous catalysis, catalysts provide a surface. Each catalyst molecule may induce the transformation of many molecules of. Catalysts Definition Chemistry.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalysts Definition Chemistry In heterogeneous catalysis, catalysts provide a surface. In other words, a catalyst is both a. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. a catalyst is some material that. Catalysts Definition Chemistry.
From exobfetmk.blob.core.windows.net
Examples Of Catalysts Gcse Chemistry at Colin Aleman blog Catalysts Definition Chemistry a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Hence a catalyst can be. catalysts allow a reaction to proceed via. Catalysts Definition Chemistry.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysts Definition Chemistry With a helping hand from a catalyst, molecules that might take years to interact can now do. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; In other words, a catalyst is both a. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than. Catalysts Definition Chemistry.
From examples.yourdictionary.com
Examples of Catalysts YourDictionary Catalysts Definition Chemistry catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. a catalyst is some material that speeds up chemical reactions. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; In other words, a catalyst is both a. a catalyst,. Catalysts Definition Chemistry.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID4503154 Catalysts Definition Chemistry With a helping hand from a catalyst, molecules that might take years to interact can now do. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. a catalyst, in turn,. Catalysts Definition Chemistry.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalysts Definition Chemistry In heterogeneous catalysis, catalysts provide a surface. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Each catalyst molecule may induce the transformation of many molecules of. Catalysts Definition Chemistry.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2684800 Catalysts Definition Chemistry In other words, a catalyst is both a. With a helping hand from a catalyst, molecules that might take years to interact can now do. a catalyst is some material that speeds up chemical reactions. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Each catalyst molecule may. Catalysts Definition Chemistry.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalysts Definition Chemistry In heterogeneous catalysis, catalysts provide a surface. In other words, a catalyst is both a. Each catalyst molecule may induce the transformation of many molecules of reactants. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. a catalyst, in turn, is a substance that is not consumed by. Catalysts Definition Chemistry.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalysts Definition Chemistry A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; In other words, a catalyst is both a. a catalyst is some material that speeds up chemical reactions. Each catalyst molecule may induce the transformation of many molecules of reactants. catalysis, the modification of the rate of a chemical reaction,. Catalysts Definition Chemistry.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts Definition Chemistry catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Each catalyst molecule may induce. Catalysts Definition Chemistry.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalysts Definition Chemistry Hence a catalyst can be. a catalyst is some material that speeds up chemical reactions. In other words, a catalyst is both a. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. catalysts allow a reaction to proceed via a pathway that has a. Catalysts Definition Chemistry.
From dxomuorny.blob.core.windows.net
Catalyst In Chemistry Examples at Alton Stonge blog Catalysts Definition Chemistry A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. In heterogeneous catalysis, catalysts provide a surface. With a helping hand from a catalyst, molecules that might take years. Catalysts Definition Chemistry.
From dxostlfbq.blob.core.windows.net
Catalytic Definition Physics at Kathleen Wilson blog Catalysts Definition Chemistry In heterogeneous catalysis, catalysts provide a surface. In other words, a catalyst is both a. a catalyst is some material that speeds up chemical reactions. Each catalyst molecule may induce the transformation of many molecules of reactants. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst, in turn,. Catalysts Definition Chemistry.
From dxoqluuit.blob.core.windows.net
Catalysts On Chemistry at Evelyn Mynatt blog Catalysts Definition Chemistry With a helping hand from a catalyst, molecules that might take years to interact can now do. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Hence a catalyst can be. Each catalyst molecule may induce the transformation of many molecules of reactants. a catalyst is some material that speeds up. Catalysts Definition Chemistry.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysts Definition Chemistry catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; In other words, a catalyst is both a. Each catalyst molecule may induce the transformation of many molecules. Catalysts Definition Chemistry.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalysts Definition Chemistry A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; a catalyst is some material that speeds up chemical reactions. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. With a helping hand from a catalyst, molecules. Catalysts Definition Chemistry.
From www.youtube.com
How does a CATALYST work ? YouTube Catalysts Definition Chemistry catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Each catalyst molecule may induce the transformation of many molecules of reactants. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In other words, a. Catalysts Definition Chemistry.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts Definition Chemistry a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.. Catalysts Definition Chemistry.
From booytrans.weebly.com
Periodic table catalyst definition chemistry booytrans Catalysts Definition Chemistry A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Hence a catalyst can be. With a helping hand from a catalyst, molecules that might take years to. Catalysts Definition Chemistry.
From www.waca.msf.org
Catalyst, Catalyst Catalysts Definition Chemistry In other words, a catalyst is both a. Each catalyst molecule may induce the transformation of many molecules of reactants. Hence a catalyst can be. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a. Catalysts Definition Chemistry.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalysts Definition Chemistry catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In other words, a catalyst is both a. Each catalyst molecule may induce the transformation of many molecules of reactants. Hence a catalyst can be. a catalyst, in turn, is a substance that is not. Catalysts Definition Chemistry.
From www.slideserve.com
PPT Role of Catalysts PowerPoint Presentation, free download ID2823169 Catalysts Definition Chemistry catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface. Each catalyst molecule may induce the transformation of many molecules of reactants. With a helping hand from a catalyst, molecules that might take years to interact can now do. a catalyst, in. Catalysts Definition Chemistry.
From www.slideshare.net
Catalyst Catalysts Definition Chemistry catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Hence a catalyst can be. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. With a helping hand from a catalyst, molecules that might take years to interact can now do. Each. Catalysts Definition Chemistry.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalysts Definition Chemistry a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Each catalyst molecule may induce the transformation of many molecules of reactants. In. Catalysts Definition Chemistry.
From www.nagwa.com
Lesson Catalysts Nagwa Catalysts Definition Chemistry catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. a catalyst is some material that speeds up chemical reactions. In heterogeneous catalysis, catalysts provide a surface. Each catalyst molecule may induce the transformation of many molecules of reactants. With a helping hand from a catalyst, molecules that might. Catalysts Definition Chemistry.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalysts Definition Chemistry a catalyst is some material that speeds up chemical reactions. In heterogeneous catalysis, catalysts provide a surface. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Hence a catalyst can be. With a. Catalysts Definition Chemistry.
From www.researchgate.net
Commonly Used Types of Catalysts and Their Range of Use Download Catalysts Definition Chemistry A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; a catalyst is some material that speeds up chemical reactions. In heterogeneous catalysis, catalysts provide a surface. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. . Catalysts Definition Chemistry.